Overview
Our dynamic Translational Chemistry platform focuses on expanding the applications of natural product-inspired compounds and leveraging these unique scaffolds to target biological systems.
We and our collaborators are currently focused on three areas for various disease indications: serotonin receptors, mitogen-activated protein kinases, and telomerase. Each of these targets is heavily involved in diseases with high societal impact, including cancer, aging, and neurological disorders. Working at the interfaces of chemistry, biology, and medicine, our past and current work showcases the promise of translational science driven by molecular concepts. Our ongoing goal is to continue developing the necessary tools to study important biological systems with the intentions of elucidating mechanisms behind disease.
Interdisciplinary Mastery
Team members who pursue research under the Translational Chemistry platform master integration of techniques available within chemistry and the life sciences. Trainees will gain knowledge in both areas of study and a unique perspective to tackle complex biological problems.
Collaboration
The integration of concepts and data across fields of study is paramount in navigating successful collaborations, which are essential in interdisciplinary study.
Synthetic Creativity
Team members will learn to combine computational chemistry and data-driven compound design to synthesize biologically active probe molecules. Creation of diverse small molecule libraries will expose trainees to a variety of synthetic techniques and promote creative thinking.
5-HT Targets
Current treatments for schizophrenia, which include typical and atypical antipsychotic drugs (APDs), are limited in efficacy, even with regard to treatment of psychosis, and often cause serious side effects. For the typical APDs, these are motor symptoms, including uncontrollable, jerky movements, prolactin elevations, and weight gain. The currently available 5-HT2C agonists, alone or as augmentation of typical and atypical APDs, have shown robust activity as antipsychotics and cognitive enhancers, without compromising motor function. A major unmet need is drugs to robustly improve psychosis and cognitive impairment of schizophrenia (CIS). We have discovered that 5-HT2C agonists, including the natural products alstonine and serpentine, can potentiate the ability of atypical APDs to ameliorate the cognitive deficit produced by sub-chronic PCP in rats and mice. Thus, 5-HT2C receptor agonism is a potential novel way to treat positive symptoms and CIS. Ultimately, this new knowledge will drive the development of new treatments for the psychosis and cognitive impairment of schizophrenia without the debilitating side effects of current treatments.
MEK Kinases
We are interested in studying the role and function of non-‘classical’ mitogen-activated protein kinase (MEK) kinases. While there have been extensive studies on the ‘classical’ MEKs (MEK1/2) which have led to FDA-approved drugs, non-‘classical’ MEKs have been less explored from the translational chemistry perspective. We are specifically interested in MEK4 and MEK7, penultimate kinases in the JNK pathway. Each of these kinases is implicated in diseases with high societal burden. We and our collaborators are currently studying MEK4/7 inhibition in the context of pancreatic cancer, acute lymphoblastic leukemia, and myocardial infarction. Through the creation of new chemical probes, we hope to gain a deeper understanding of the roles of MEK4 and MEK7, which in turn will provide proof-of-principle and precedence for novel targeted therapies against these diseases.
Telomerase
Telomerase is a ribonucleoprotein enzyme that plays an important role in the immortalization of cancer cells. Normally, the shortening of telomeres during successive rounds of DNA replication eventually leads to the cessation of cell division, limiting the proliferative potential of eukaryotic cells. While telomerase is not activated in most normal somatic cells, 80-90% of primary tumor cells show telomerase activity in humans. Furthermore, telomerase has demonstrated to have multiple oncogenic and tumorigenic roles outside of its role in telomere maintenance, demonstrating the potential for telomerase as a therapeutic target in numerous cancer types. Inspired by the natural product chrolactomycin, a reported telomerase inhibitor, we have undertaken a program to design, synthesize, and evaluate novel small molecule inhibitors of telomerase.
Latest Publications
The Scheidt lab has a strong emphasis on creative problem-solving and a commitment to mentorship.
View All Publications ›
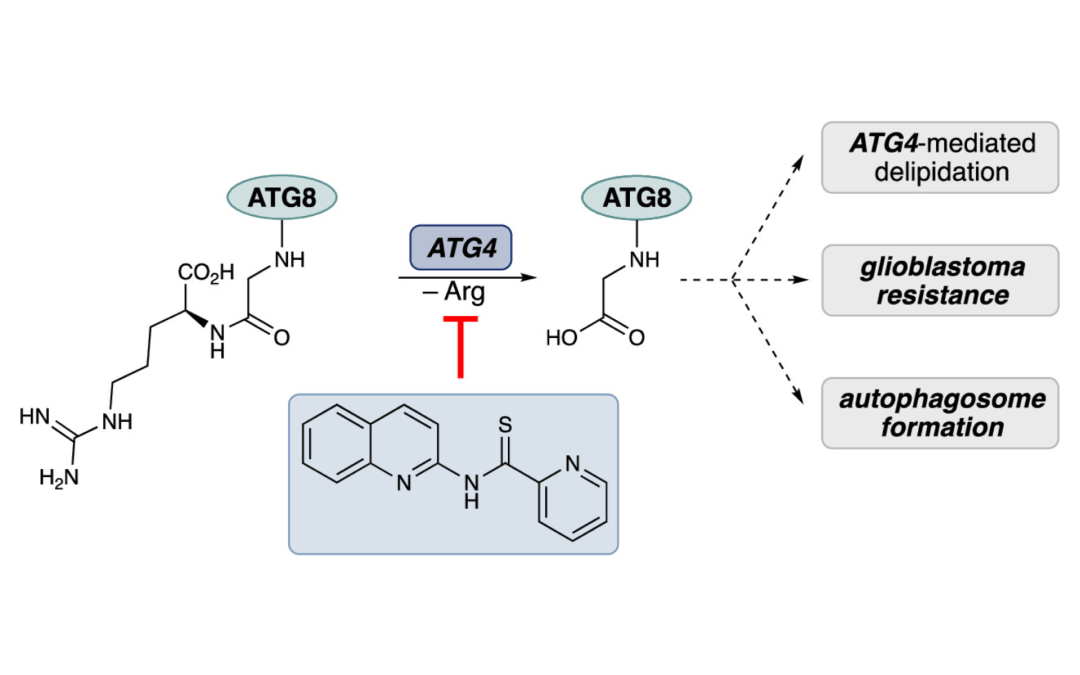
Synthesis and Structural Optimization of ATG4B Inhibitors for the Attenuation of Autophagy in Glioblastoma
Kim. D. R.; Orr, M. J.; Yu, X.; Munshi, H. H.; Wang, A.; Trudeau, C.; Kwong, A. J.; Cheng, S.-Y.; Scheidt, K. A.* ACS Med. Chem. Lett. 2024, 15, 258-264.
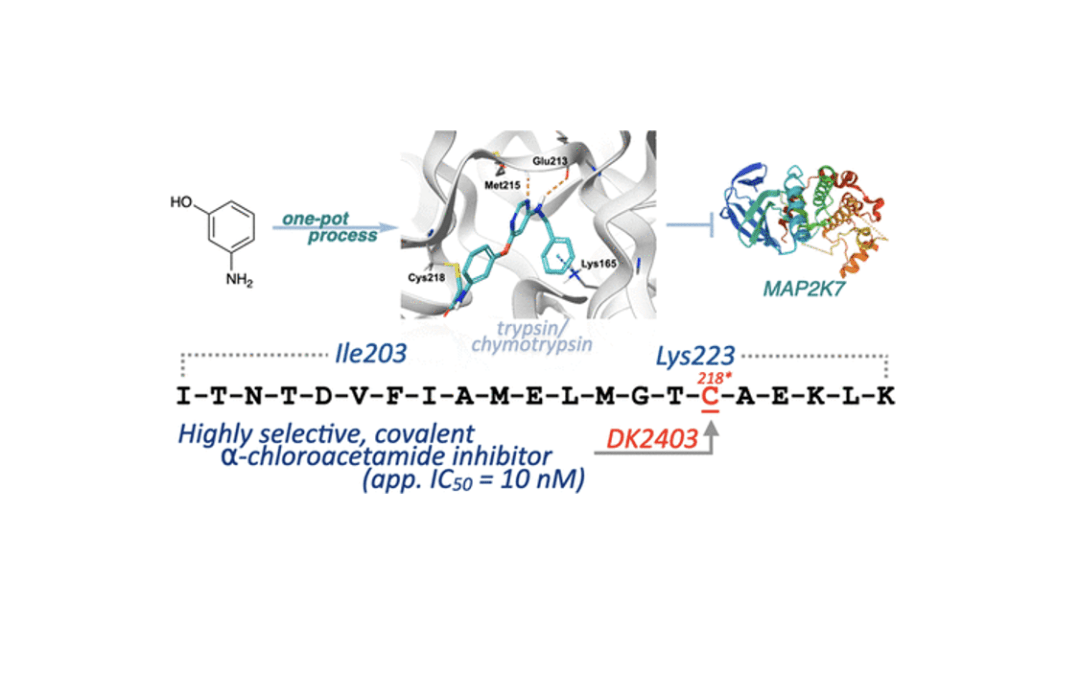
Rational Design of Highly Potent and Selective Covalent MAP2K7 Inhibitors
Kim, D. R; Orr, M. J.; Kwong, A. J.; Deibler, K. K.; Munshi, H. H.; Bridges, C. S; Chen, T. J.; Zhang, X.; Lacorazza, H. D.; Scheidt, K. A.* ACS Med. Chem. Lett. 2023, 14, 606-613.
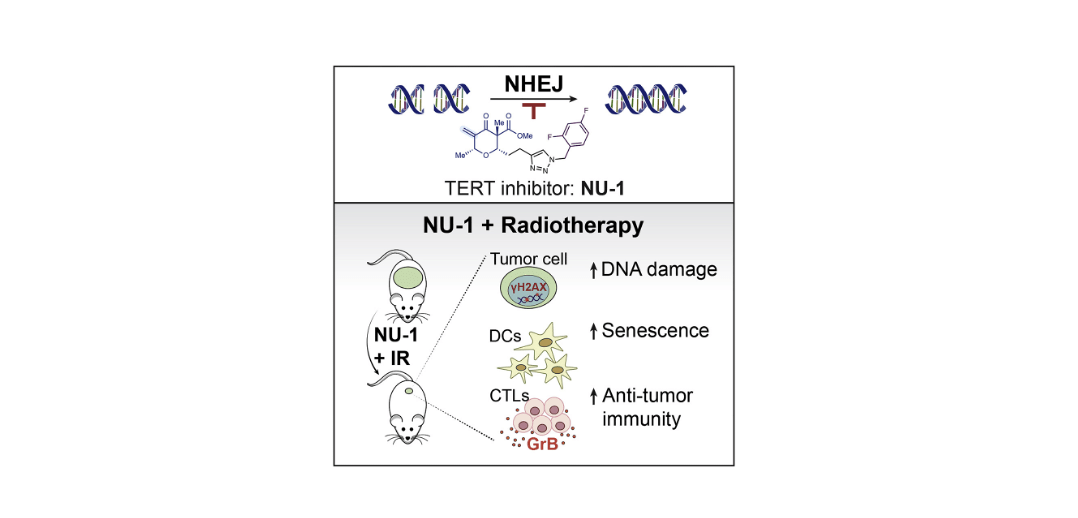
Targeting telomerase reverse transcriptase with the covalent inhibitor NU-1 confers immunogenic radiation sensitization
Liu, Y.; Betori, R. C.; Pagacz, J.; Frost, G. B.; Efimova, E. V.; Wu, D.; Wolfgeher, D. J.; Bryan, T. M.; Cohen, S. B.; Scheidt, K. A.; Kron, S. J.* Cell Chem. Biol. 2022, 29, 1517-1531.


