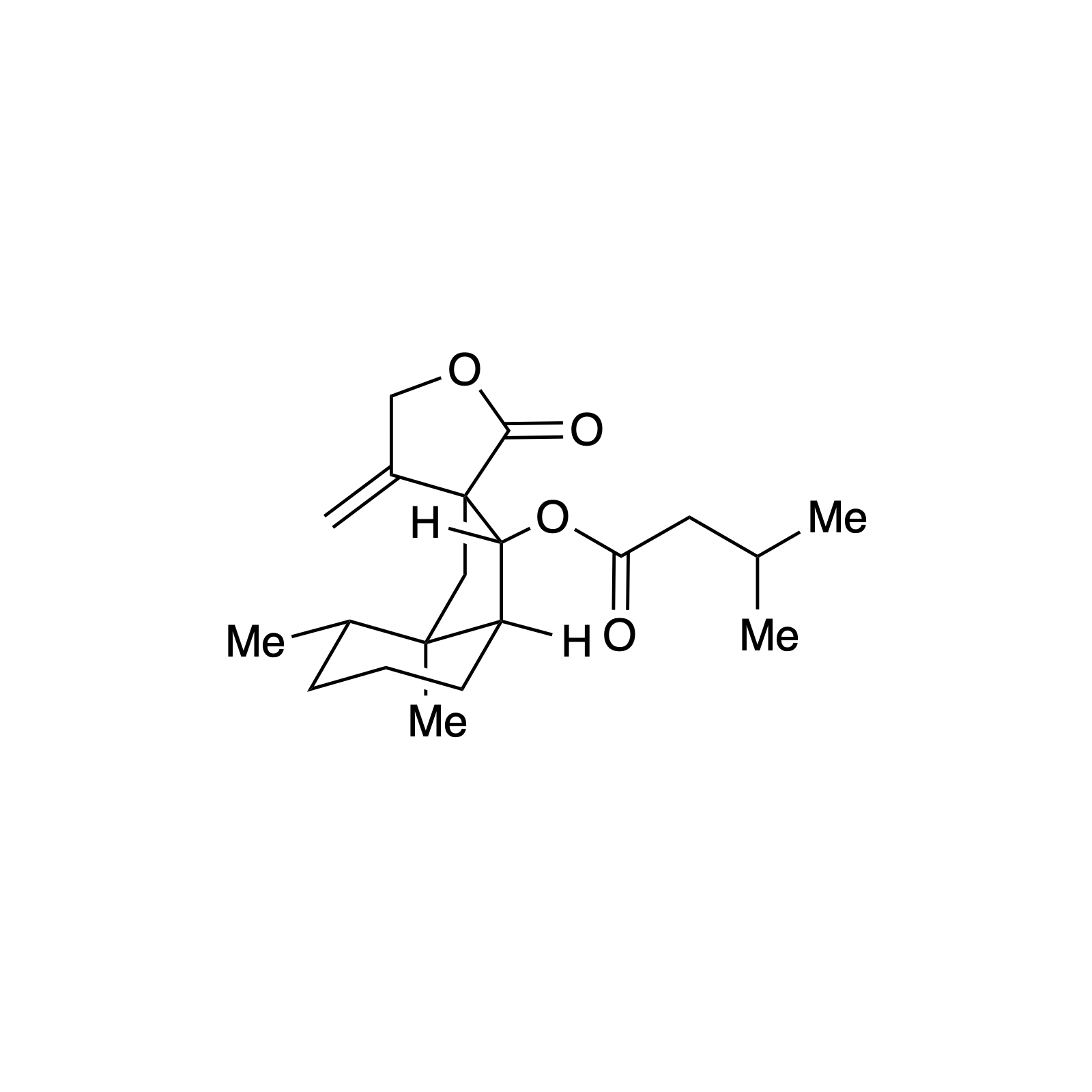
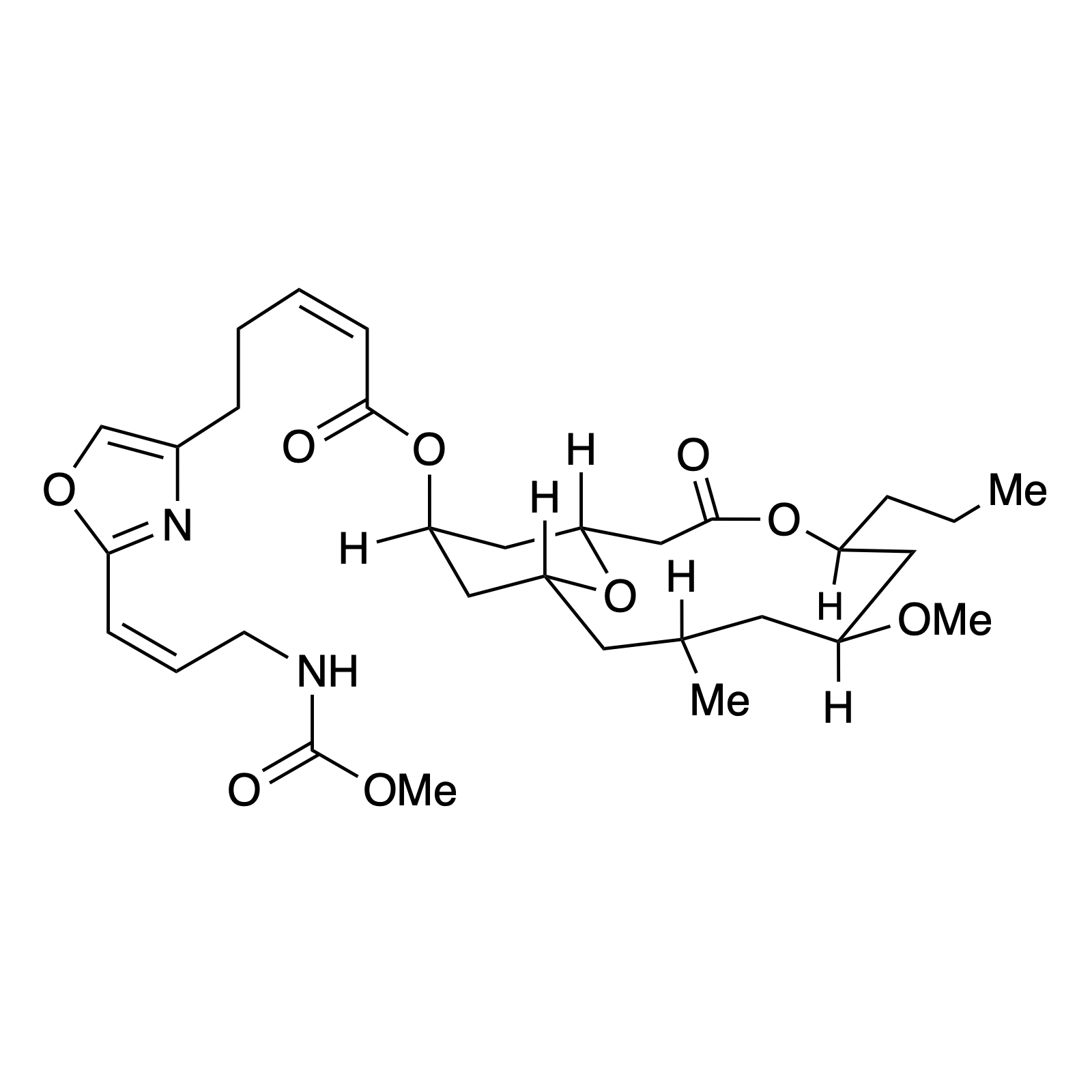
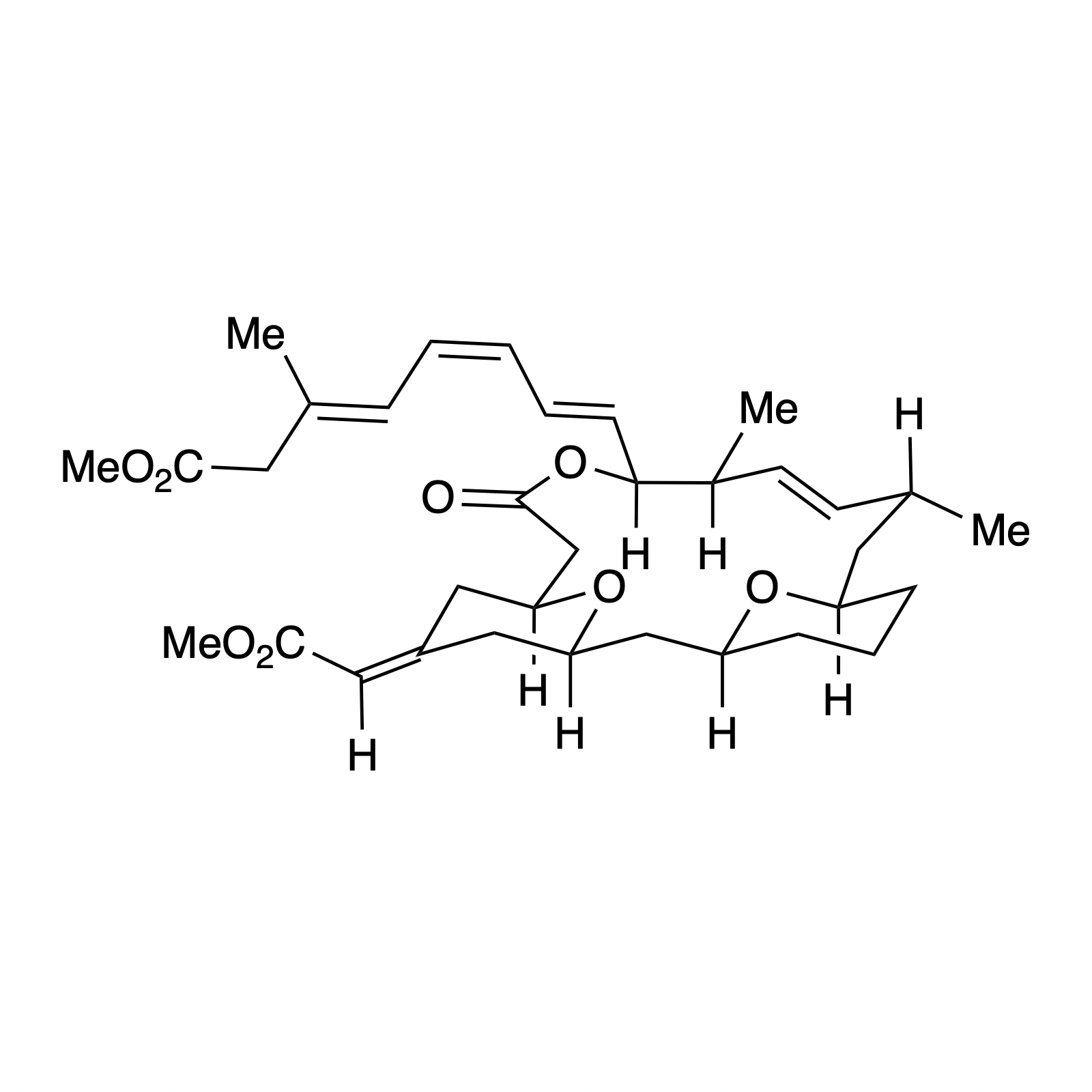
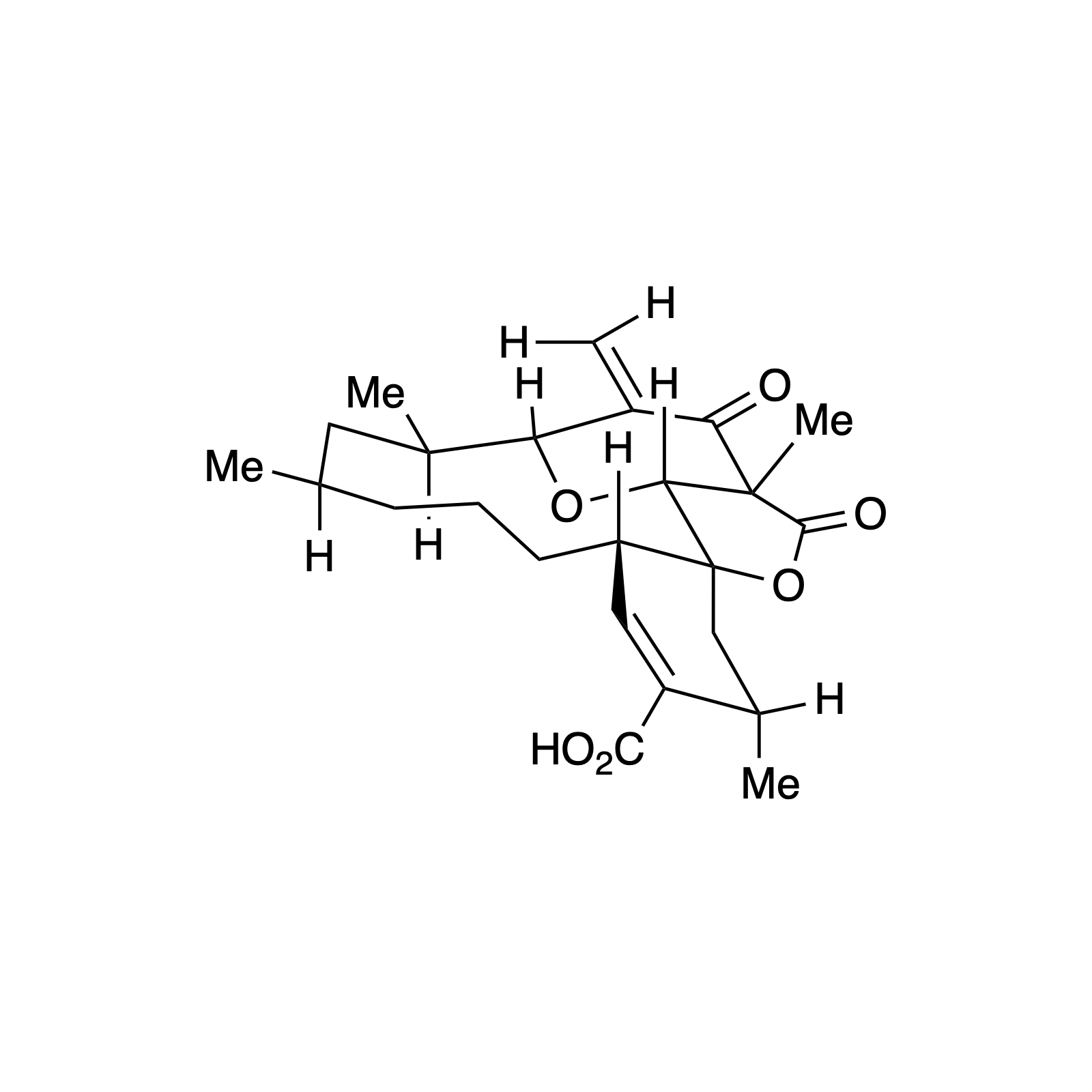
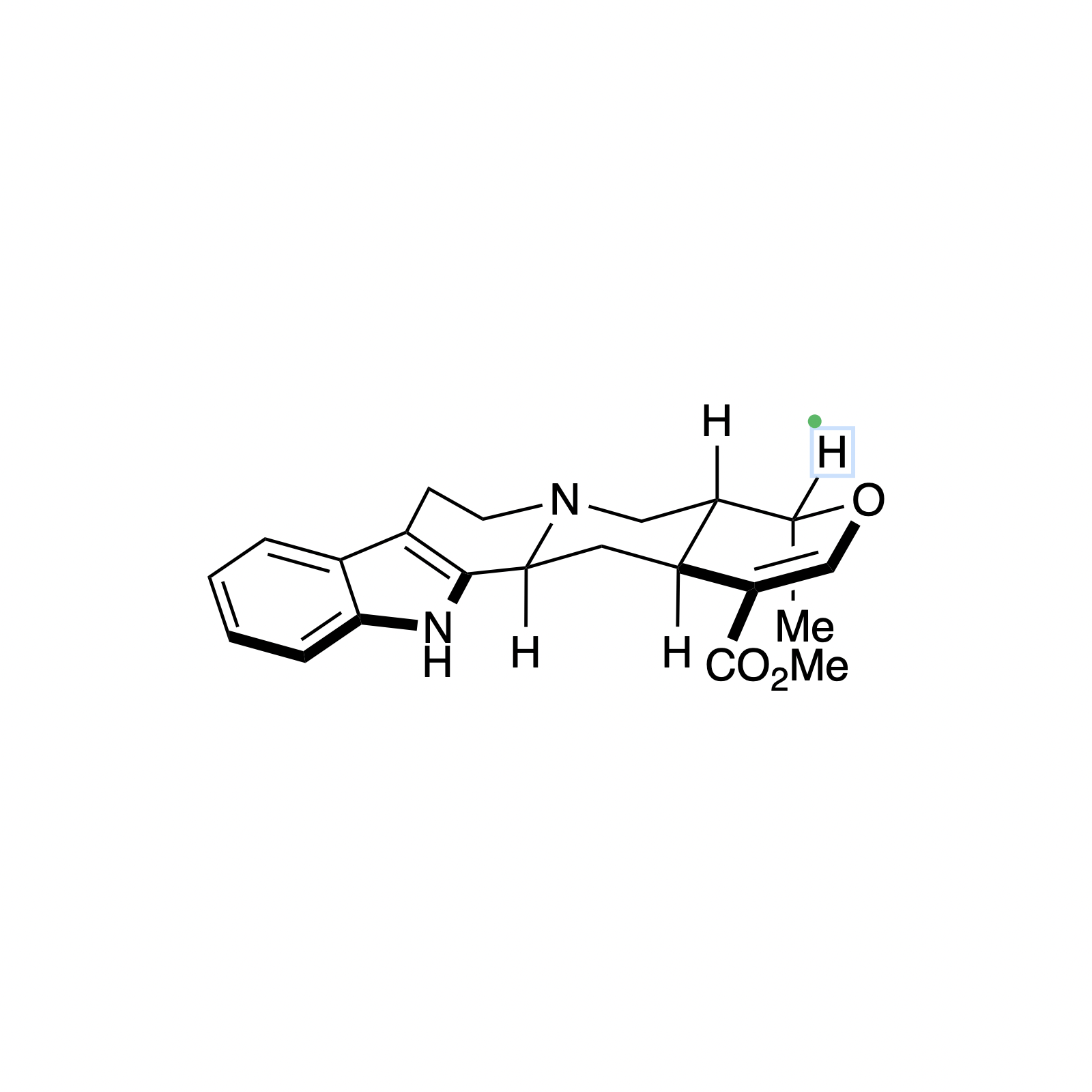Overview
Our Complex Synthesis platform is where strategic planning meets practical execution. The ability to design and conduct syntheses of architecturally complex and biologically active small molecules is invaluable for both scientific and personal advancement.
The development of concise, enantioselective syntheses generates an enabling platform to access and investigate the therapeutic potential of scarcely available natural materials and structurally related analogs. This practice provides the practitioner with a powerful skill set in synthetic planning, problem solving within complex systems, and creative thinking. This journey provides preparation for any career path, from medicinal chemistry and chemical development to materials science. A few of our recent accomplishments are detailed below.
Application-driven Synthesis
To develop concise, enantioselective syntheses of biologically active small molecules to facilitate investigation of their therapeutic potential
Novel Methodology Applications
To apply new bond-forming methodologies and strategies that expedite the synthesis of complex natural products
Critical Thinking and Problem Solving
To provide students with a powerful skill set in synthetic problem solving, creative thinking, and a deep understanding of all factors contributing to reactivity and selectivity within a given system
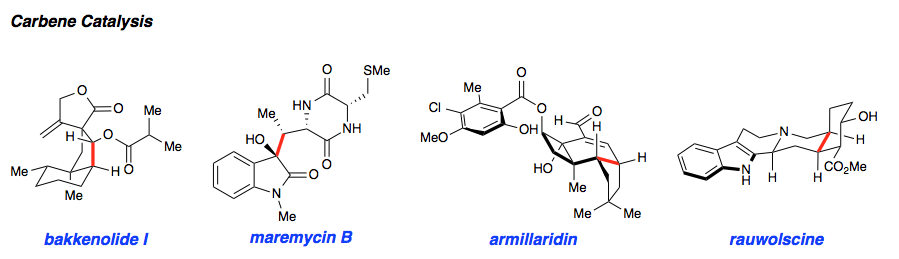
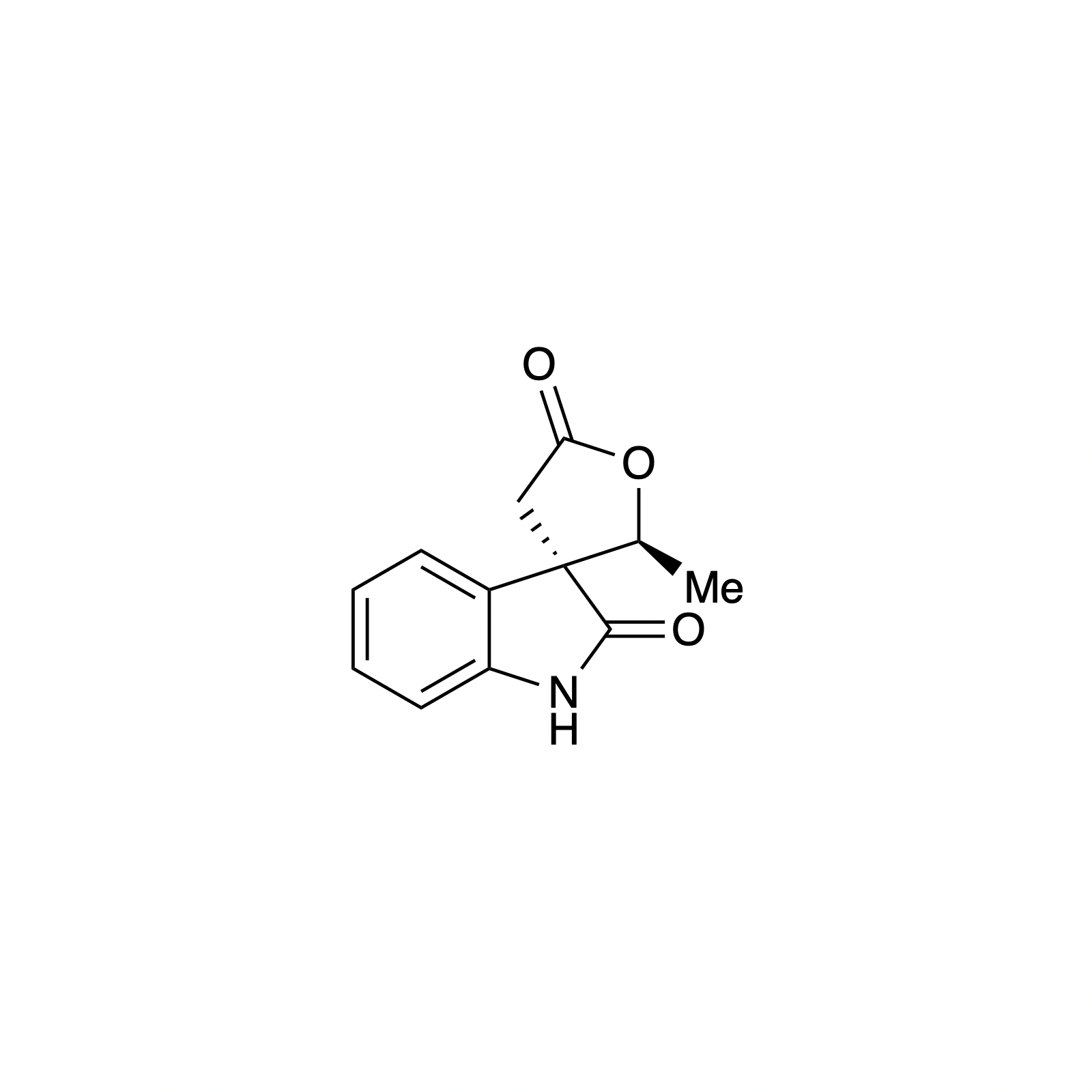
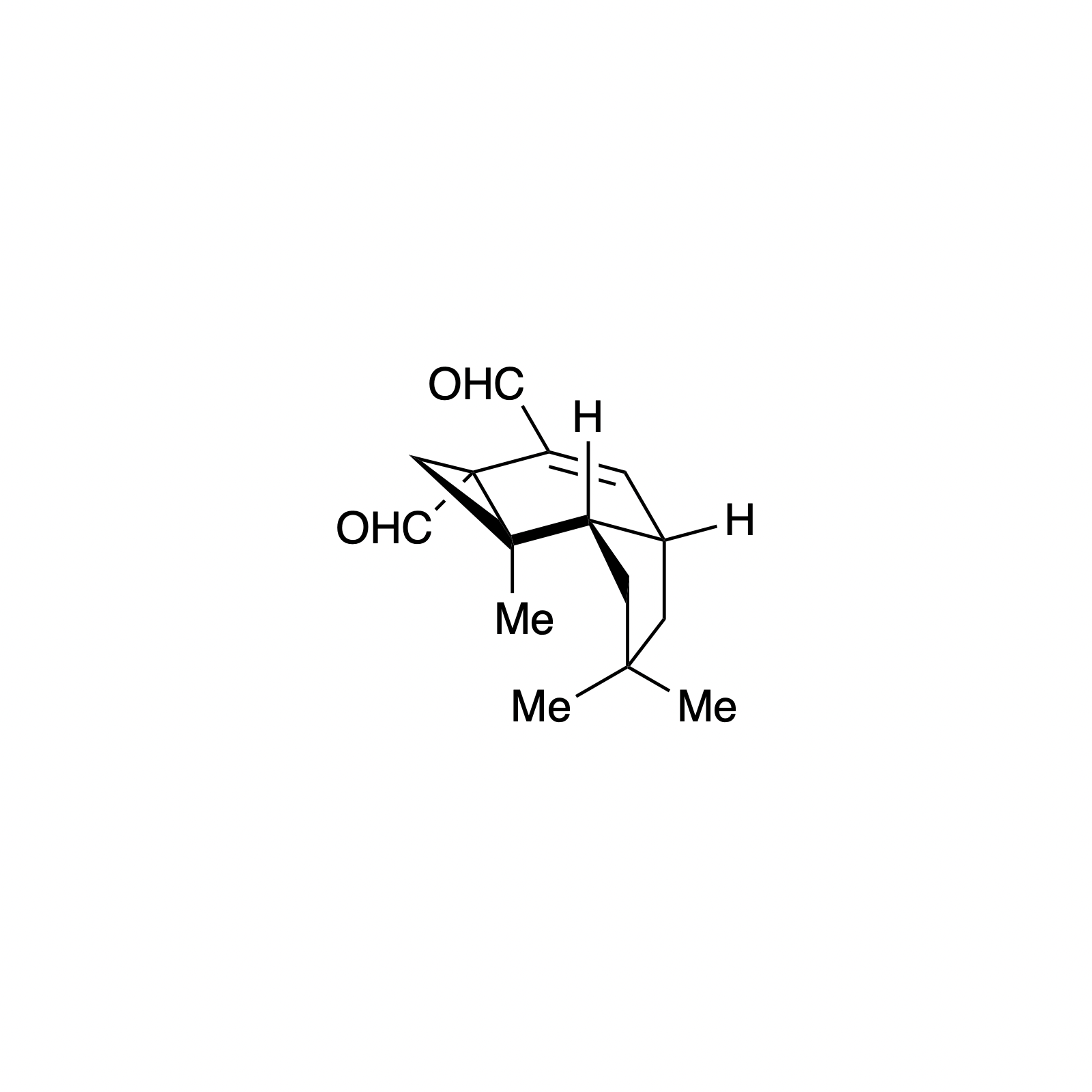
Carbene Catalysis
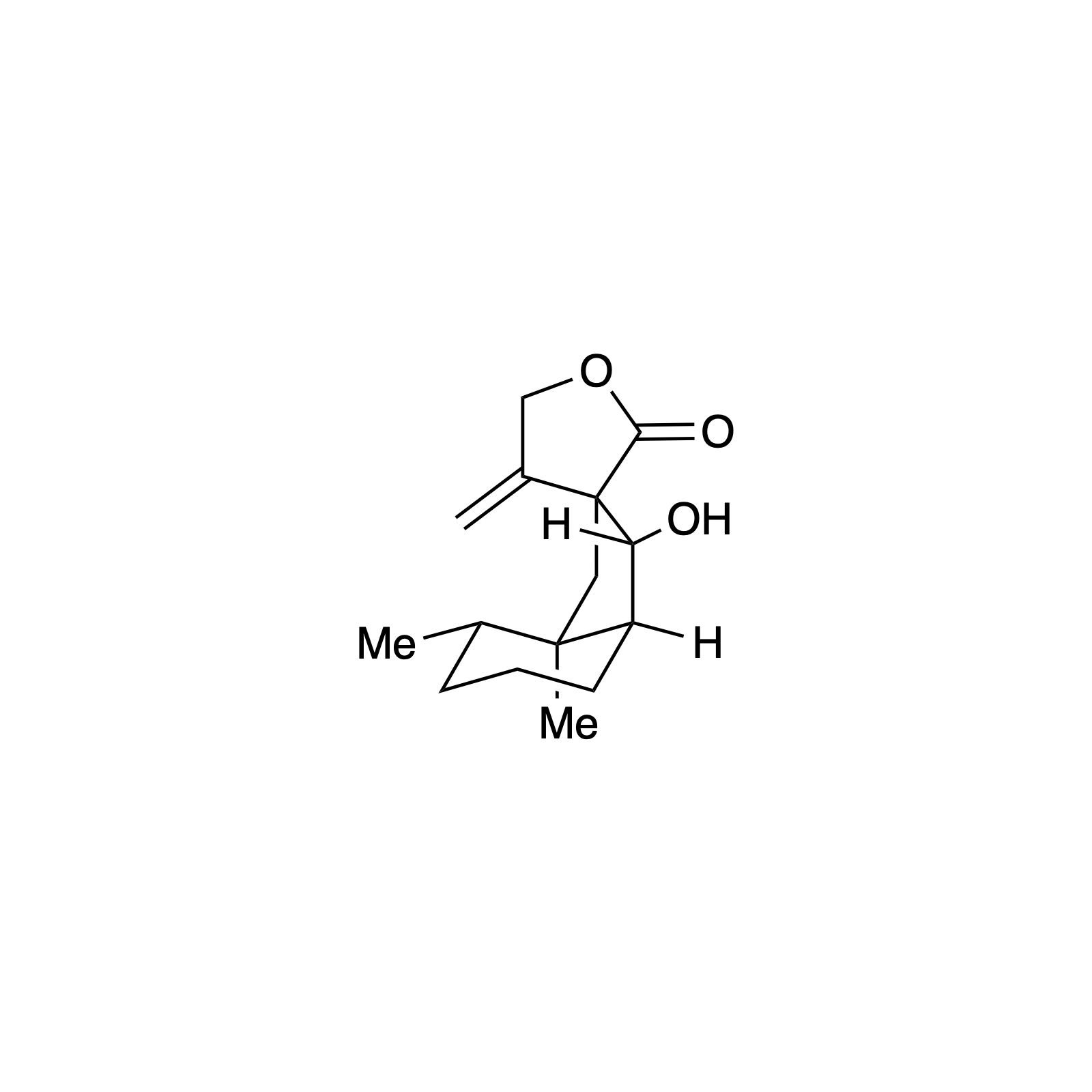
N-heterocyclic carbene (NHC) organocatalysis has proven to be a powerful platform for the synthesis of enantioenriched carbocycles and heterocycles. As such, we have been interested in applying NHC catalysis to expedite the asymmetric synthesis of complex and biologically active small molecules. Using carbene catalysis, the hydrindane core of the bakkenolides was constructed with high levels enantioselectivity and diastereoselectivity using an NHC-mediated aldol reaction. The hydrindane system of echinocidin B and D, isovelleral, and armillaridin was constructed in an analogous manner using an NHC-catalyzed Michael addition. In our concise synthesis of the yohimbine alkaloid rauwolscine, the piperidine core was constructed in high selectivity by an NHC-promoted dimerization reaction.
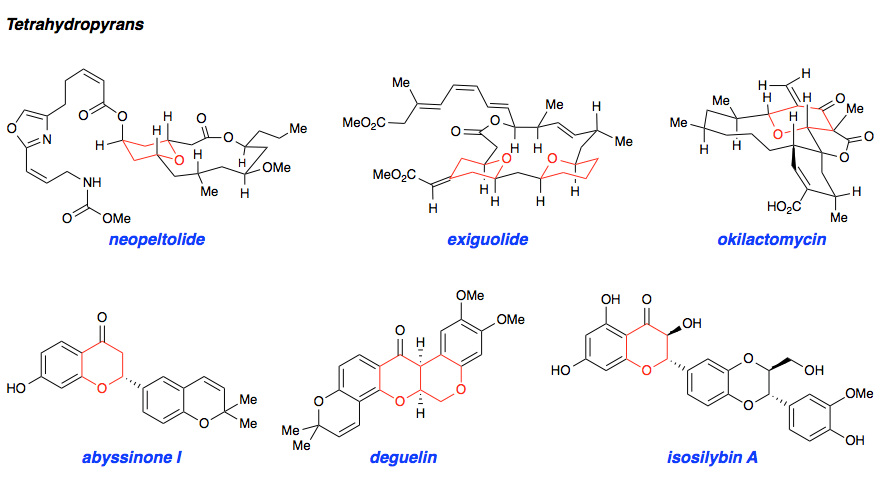
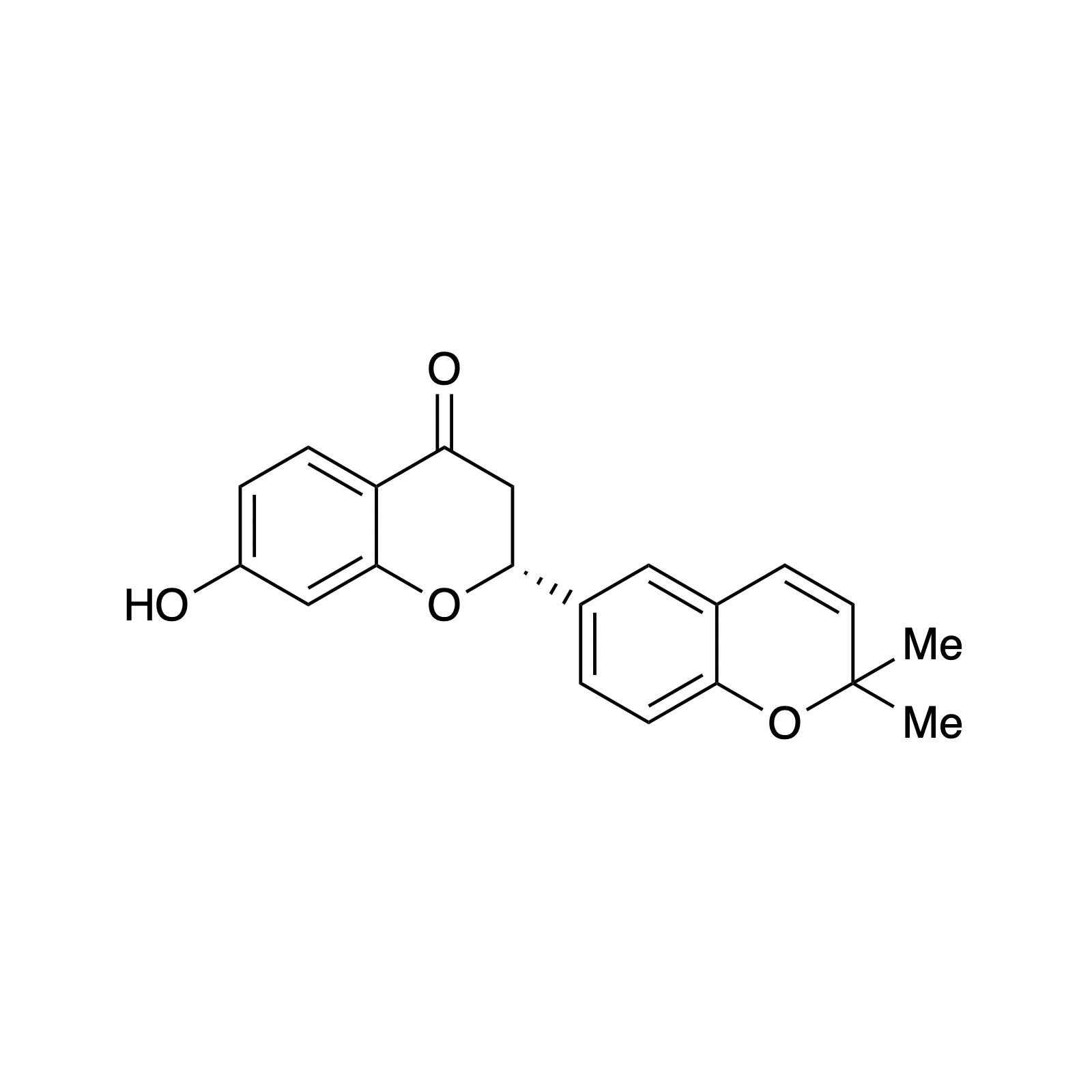
THPs
Our interest in targets containing substituted tetrahydropyran units inspired the development of a new methodology involving a one pot, highly diastereoselective Sc(III)-catalyzed synthesis of 2,6-cis-disubstituted tetrahydropyran rings. We demonstrated the powerful bond-forming ability of this strategy in our syntheses of neopeltolide and (–)-exiguolide, in which the key bond-forming steps involved concurrent formation of the pyran ring and closure of the macrocycle. This method was further modified to a Prins-type Maitland-Japp reaction, enabling the synthesis of okilactomycin. Biological studies were performed on both neopeltolide and (–)-exiguolide, revealing interesting mechanistic insights of these potential chemotherapeutics.
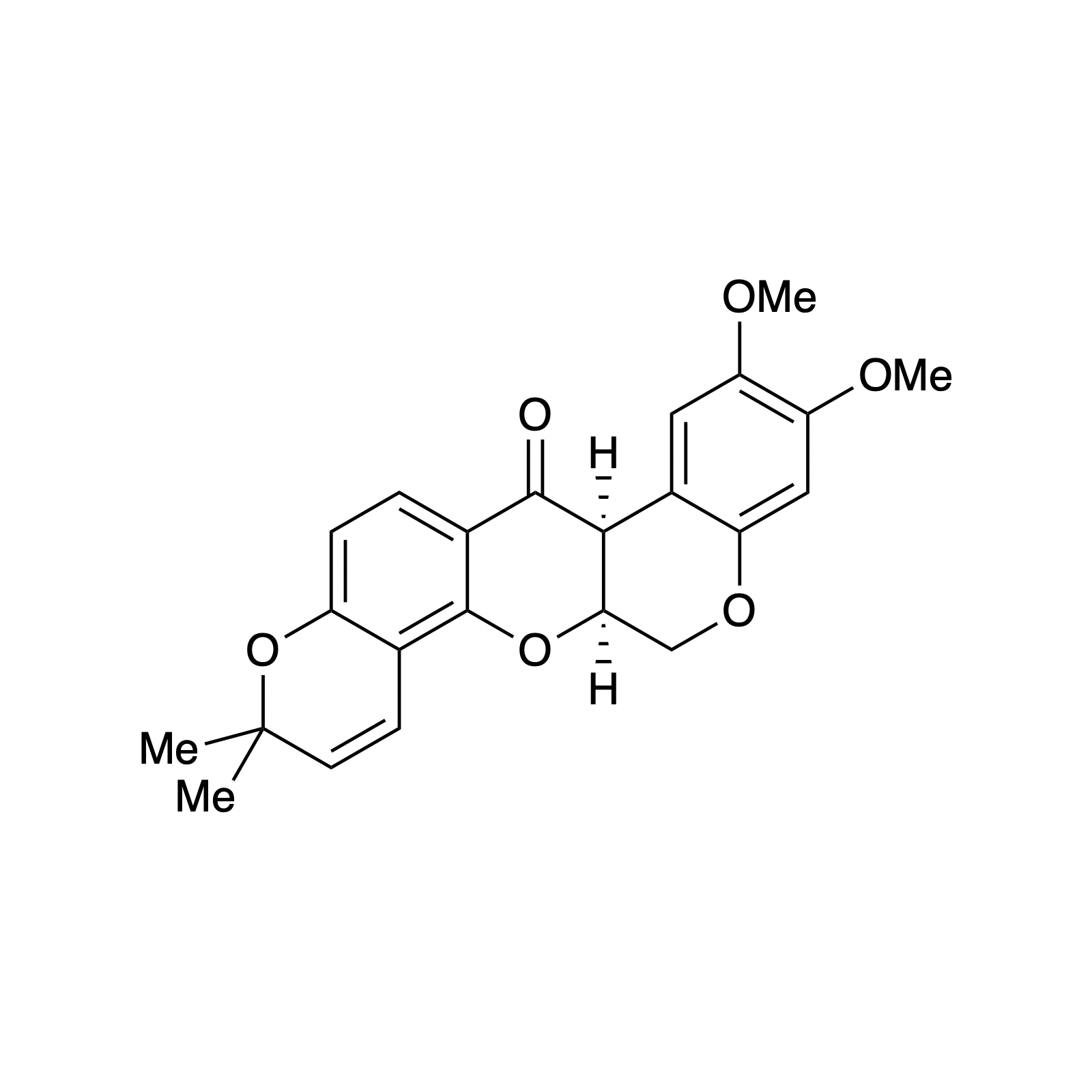
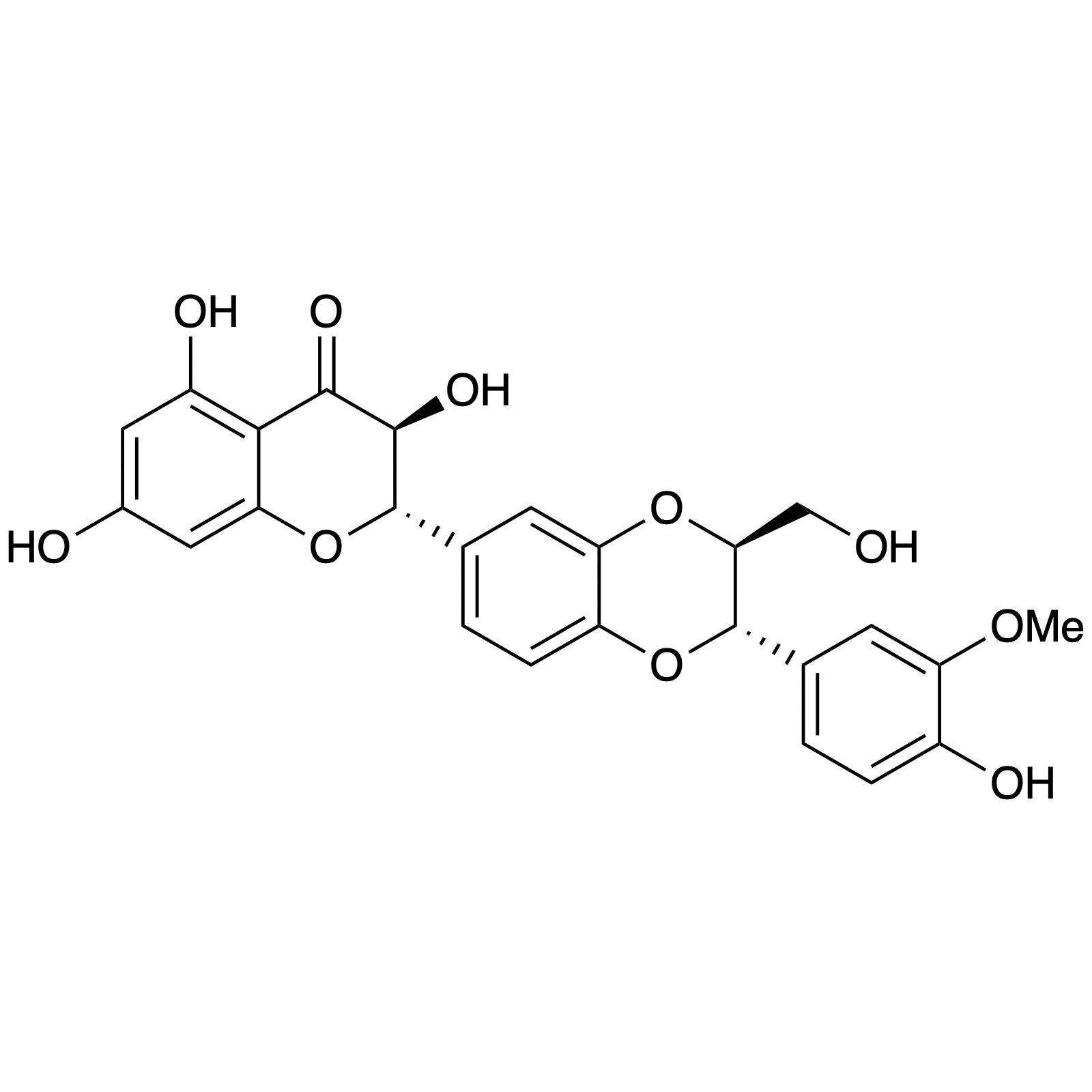
Complementary to our interest in forming highly functionalized pyranones, we have also pursued the catalytic asymmetric synthesis of benzopyranones, the core structures of flavonoids. This class represents a broad collection of plant secondary metabolites that possess a diverse array of biological activity and medicinal applications, particulary as anti-cancer therapeutics. We sought to mimic the biosynthesis of these compounds using a bifunctional H-bonding/ Brønsted base catalyst as a small molecule analog of the enzyme chalcone isomerase. This strategy was demonstrated using simple alkylidene malonates and then extended to the synthesis of (–)-deguelin, (–)-isosilybin A, and the abyssinone family of natural products.
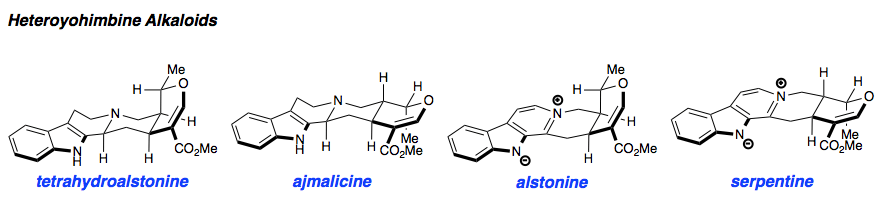
Heteroyohimbine Alkaloids
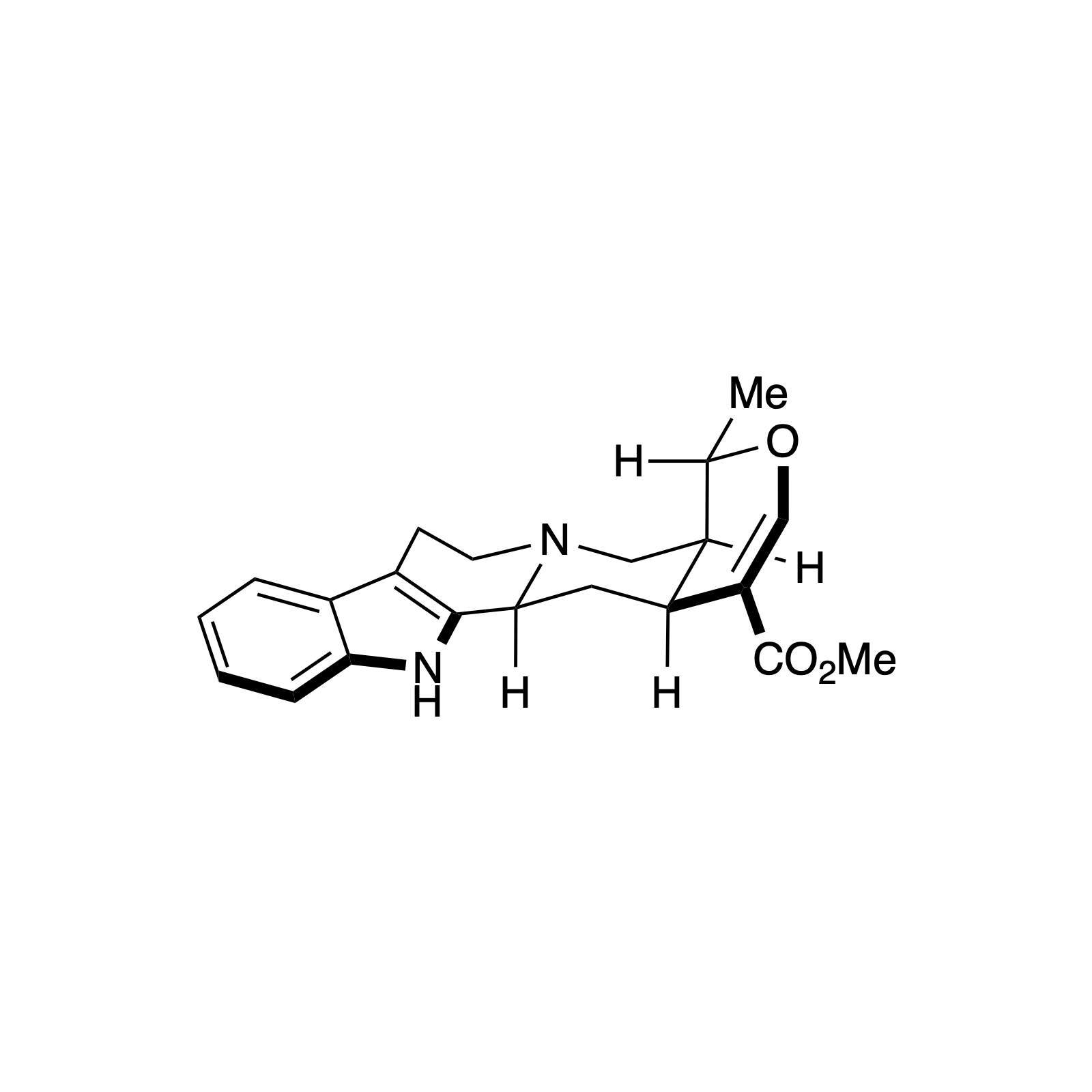
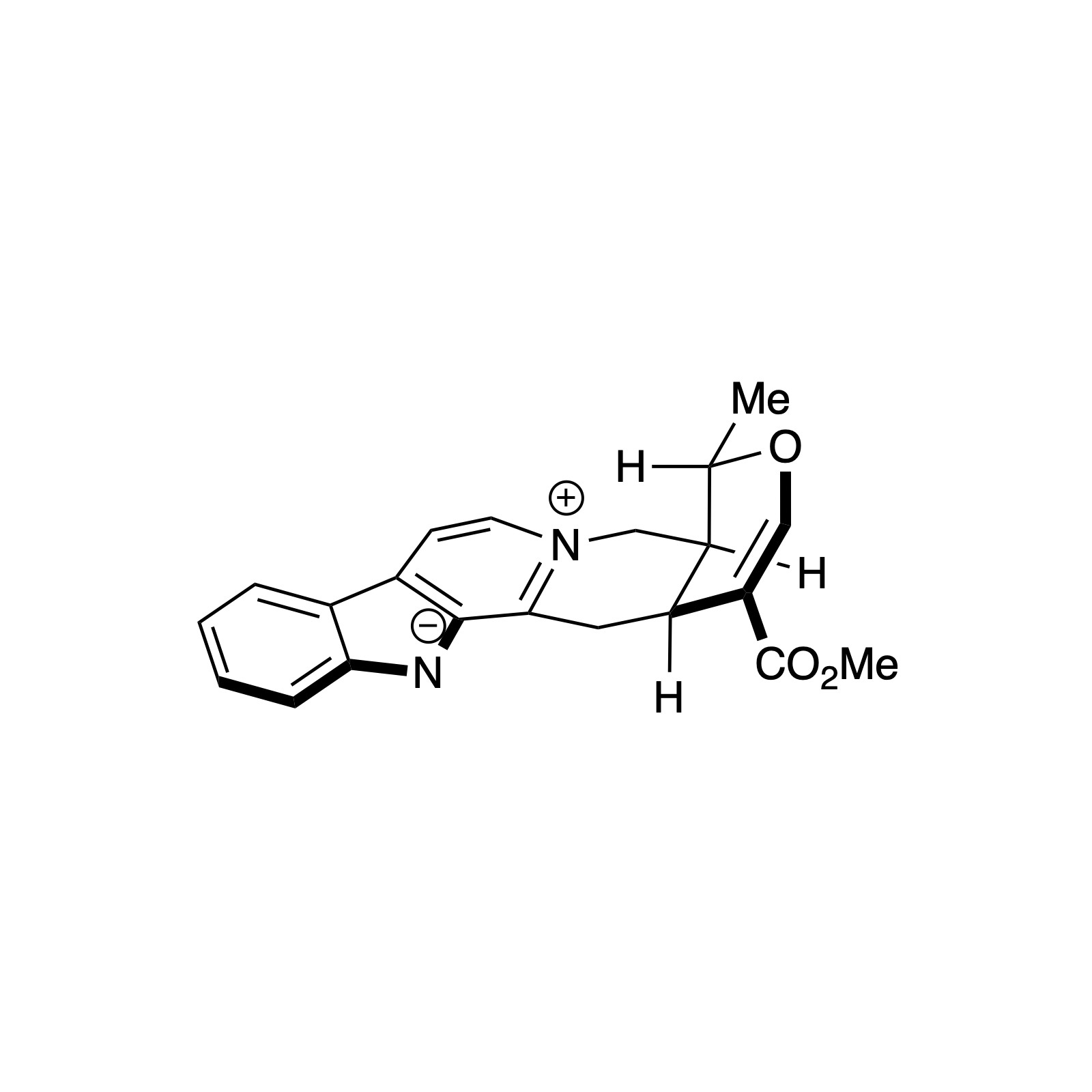
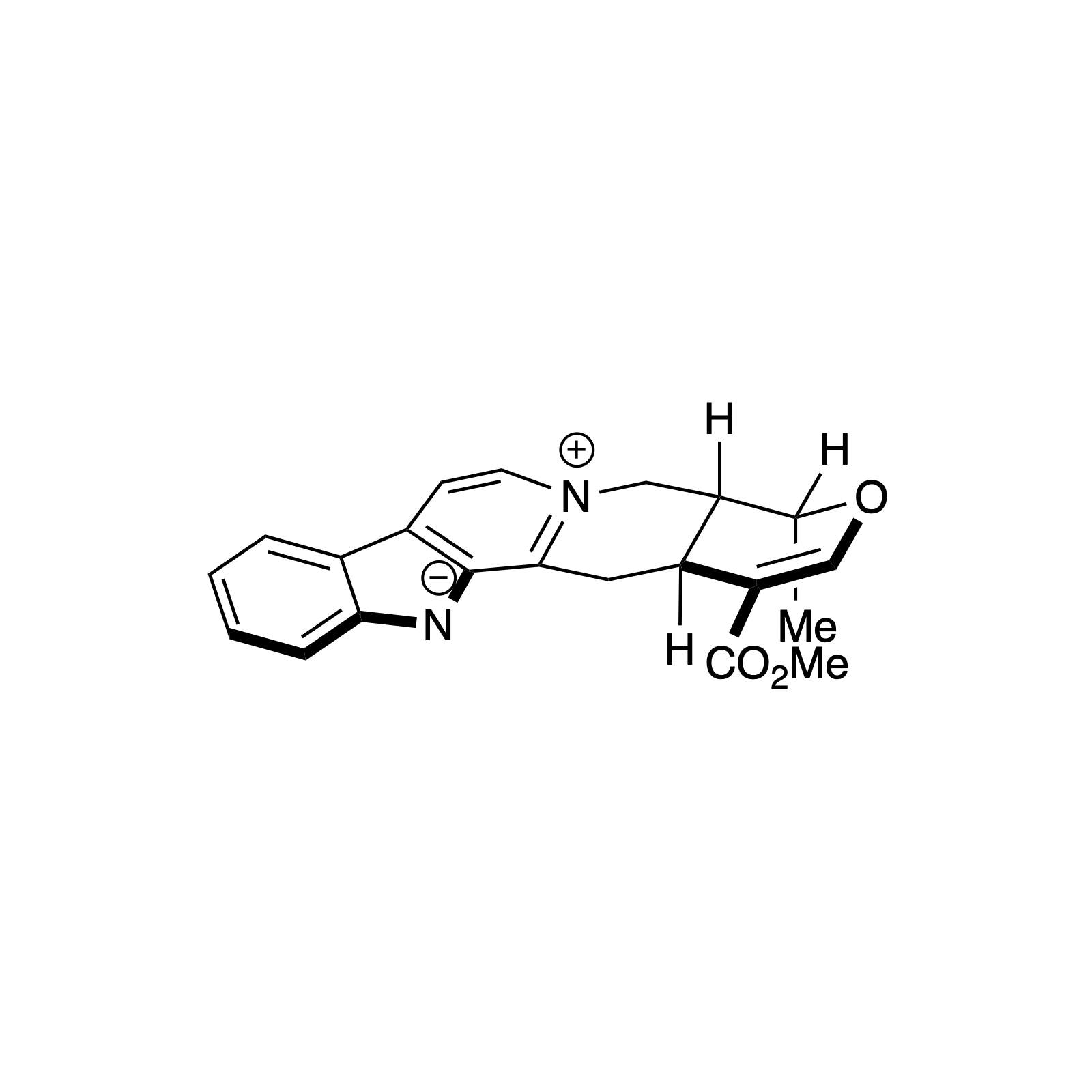
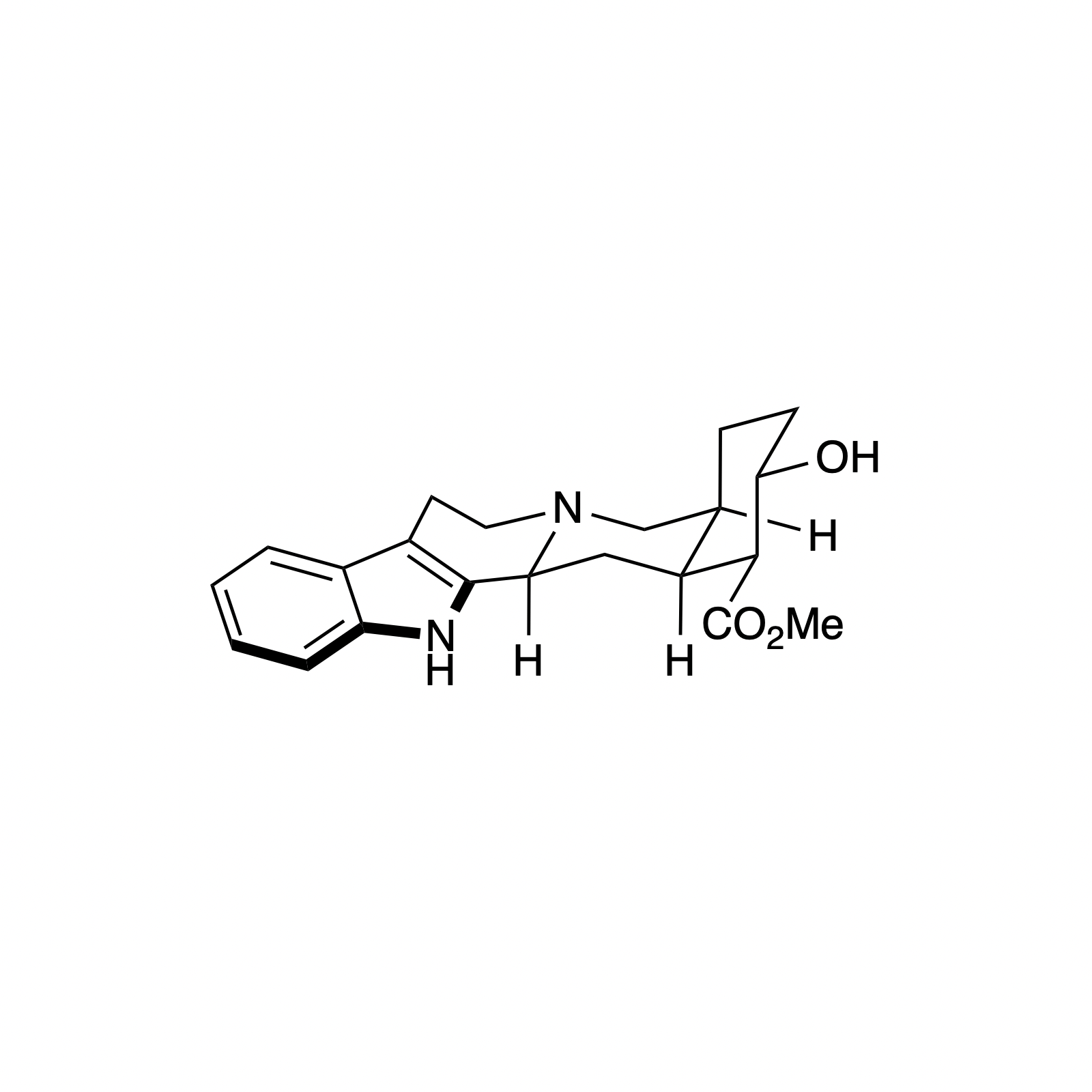
Alstonine has recently been identified as the major component of a plant-based treatment used in Nigeria by traditional healers to treat psychotic disorders. However, the scarcity and lack of purity from natural sources, as well as the uncertainty regarding its exact mechanism of action drove our interest in the asymmetric synthesis of these natural products. The related trans diastereomer, serpentine, exhibits anticancer and antimalarial properties, and there have been limited efforts at elucidating its mechanism of action. We have recently accomplished the first enantioselective total syntheses of alstonine and serpentine with a novel cooperative H-bond donor/enanmine catalysis reaction as the key step. This synthetic route has allowed us to construct a medicinal chemistry platform to investigate the biological activity of related analogues (see Bioorganic Section).
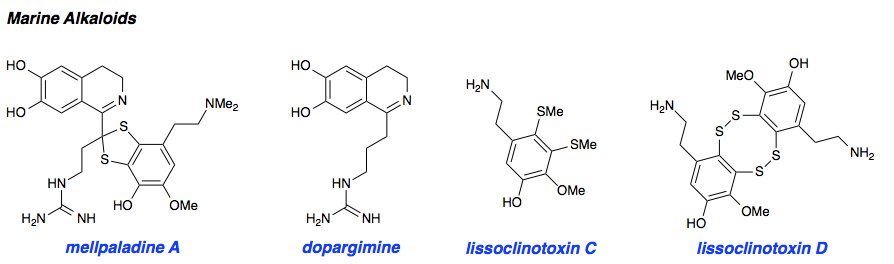
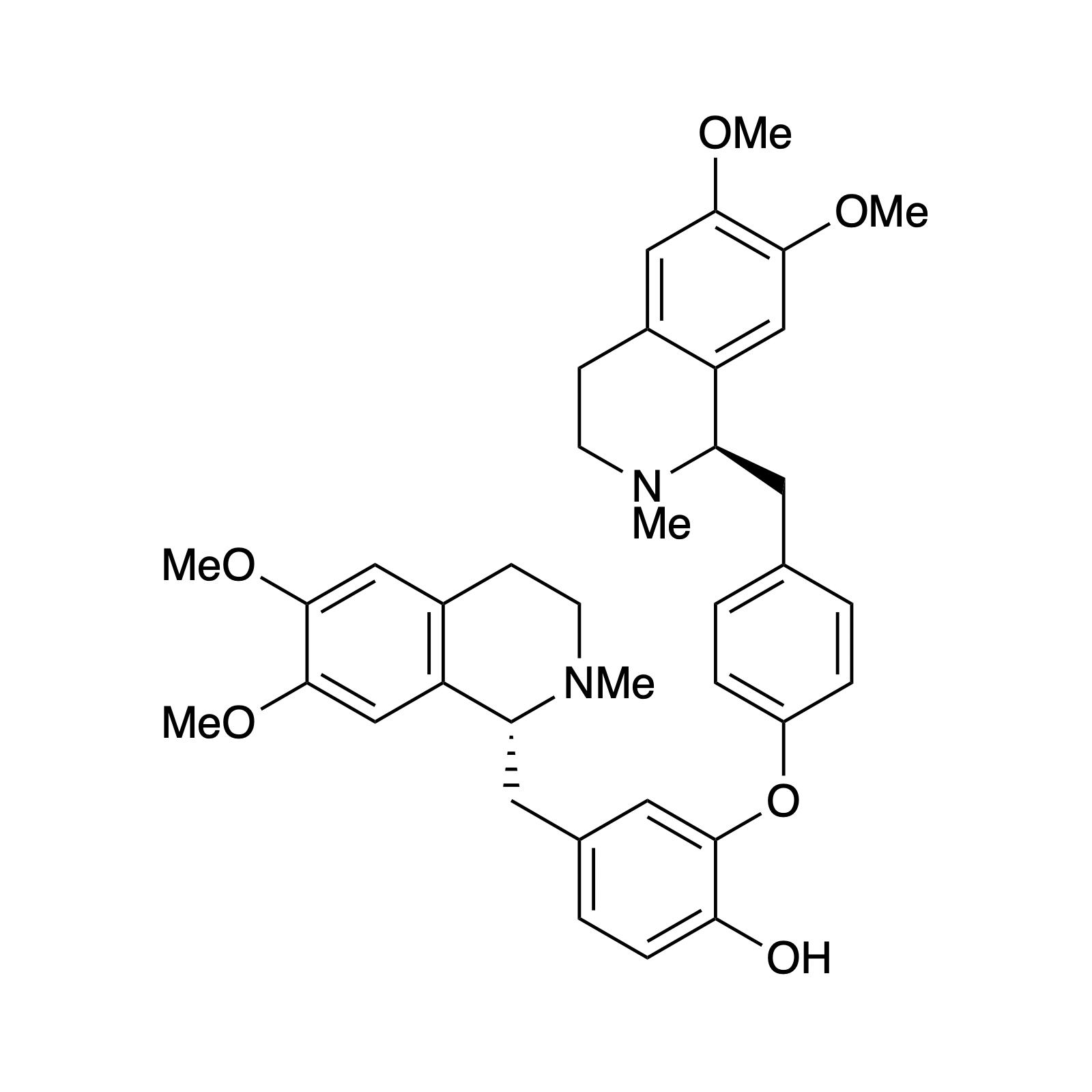
Marine Alkaloids
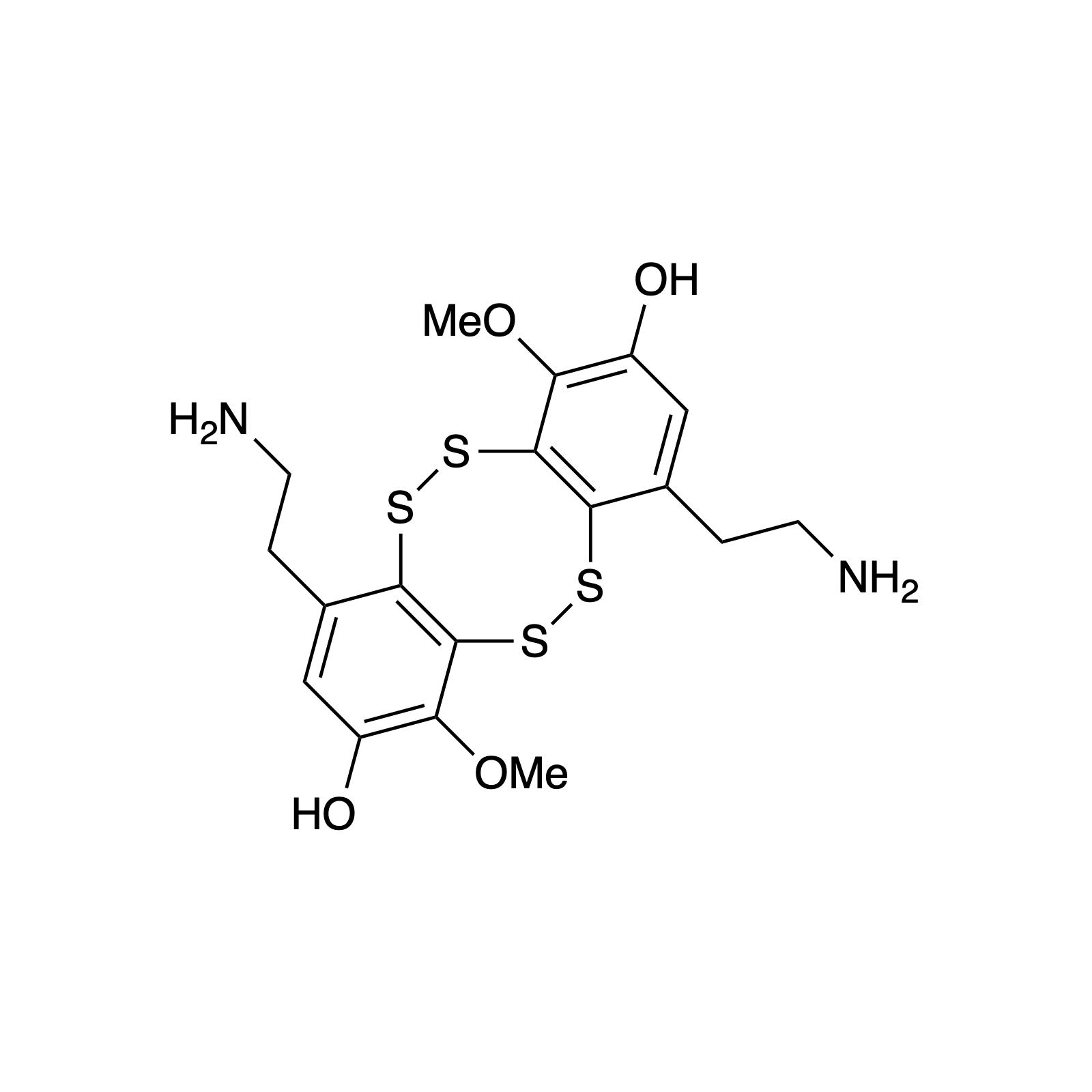
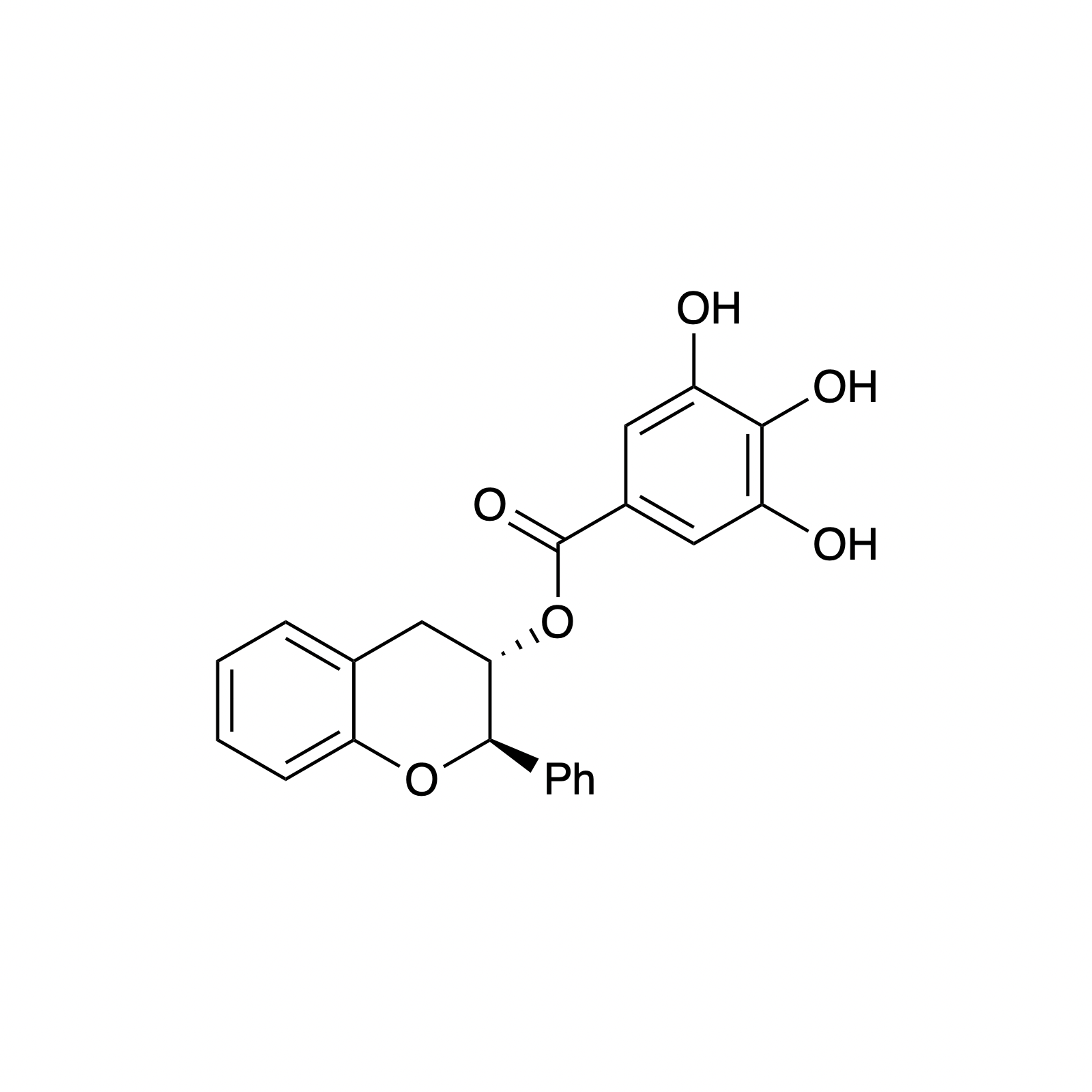
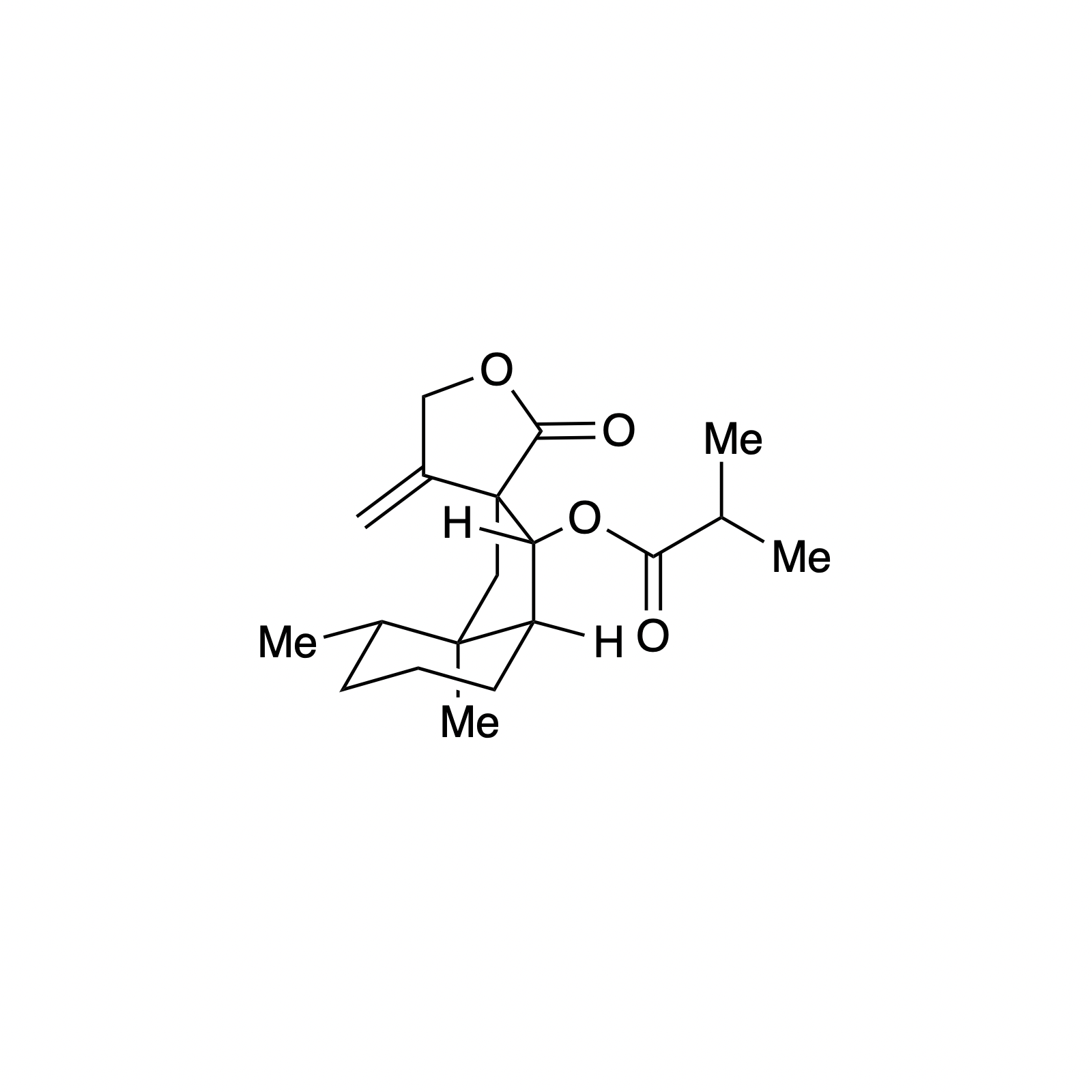
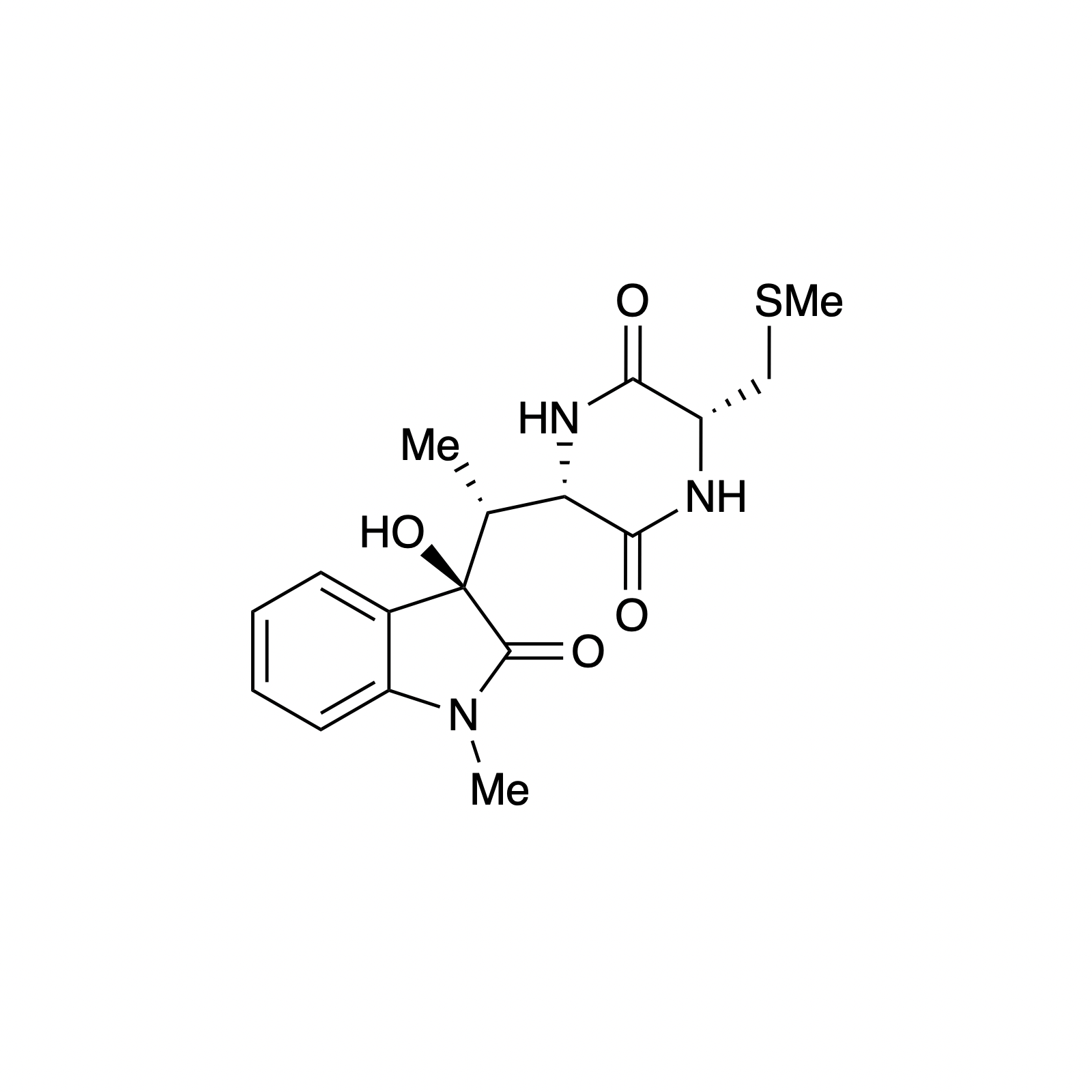
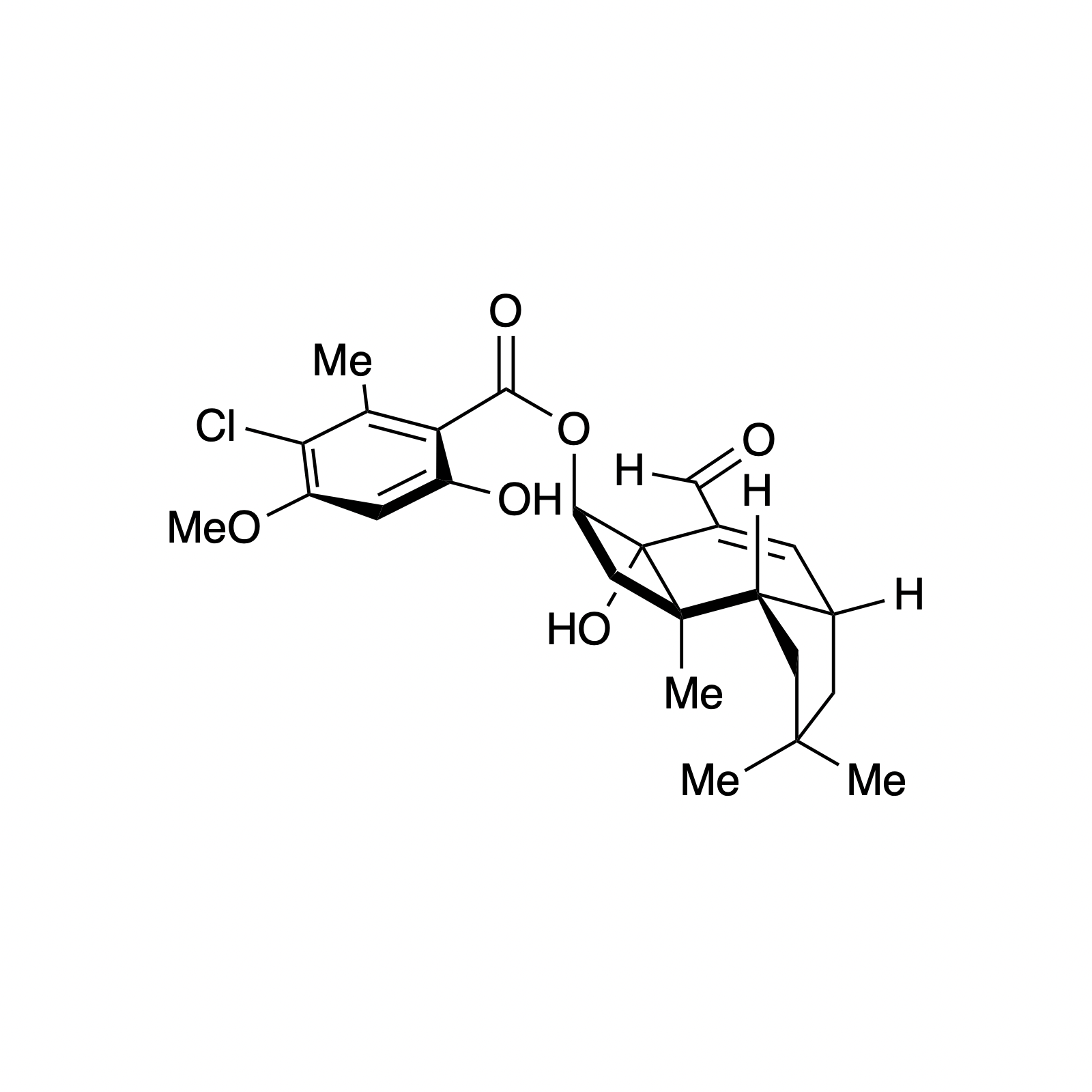
Mellpaladines A and B are sulfurous guanidine alkaloids isolated from a Palauan tunicate of the family Didemnideae in 2016. Each of these polycyclic dopamine derivatives contains a unique 1,3-benzodithiole moiety, differentiating them from all other known natural products. The mellpaladines exhibit potent and selective 5HT5A antagonism, rendering them highly desirable neuropharmacological probes. This receptor is thought to be implicated in a variety of mood disorders, and studies have demonstrated a correlation between 5HT5A polymorphisms and schizophrenia. To date, the efficacy of 5HT5A antagonism in these contexts remains unknown due to a dearth of selective inhibitors. Central to our synthesis of mellpaladine A was the condensation of an elaborated 1,2-arenedithiol with α,α-dichloro-γ-butyrolactone to form a key spirocyclic intermediate. This approach may be expanded towards the rapid synthesis of a variety of benzodithioles, thus enabling access to a unique potential pharmacophore. Other methodology developed for the synthesis of mellpaladine A has been applied towards the preparation of dopargimine as well as lissoclinotoxins C and D.
Latest Publications
The Scheidt lab has a strong emphasis on creative problem-solving and a commitment to mentorship.
View All Publications ›
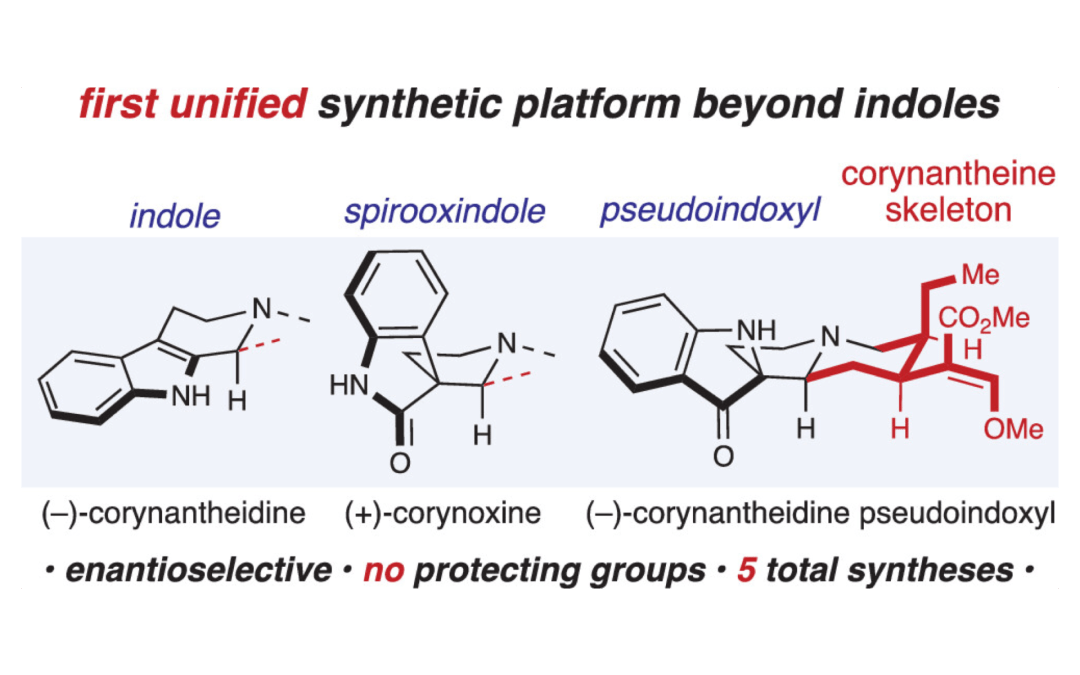
A Platform for the Synthesis of Corynantheine-Type Corynanthe Alkaloids
Nam, Y.; Tam, A. T.; Miller, E. R.; Scheidt, K. A.* J. Am. Chem. Soc. 2024, 146, 118-124.
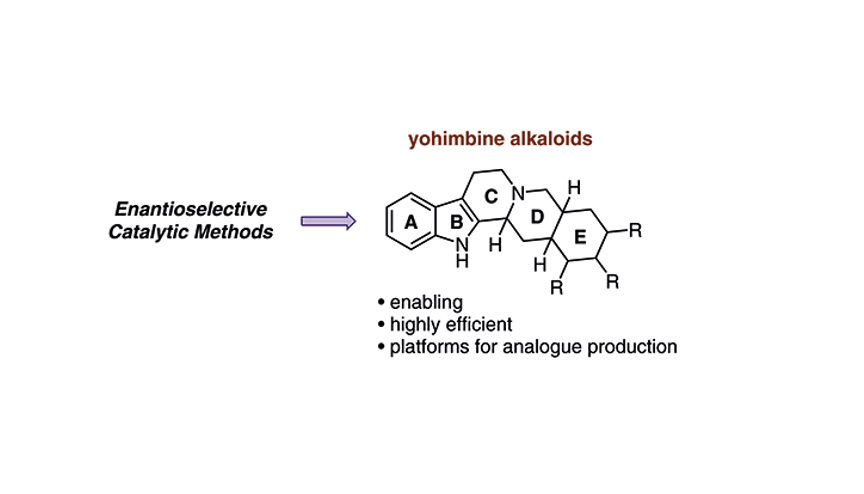
Enantioselective Syntheses of Yohimbine Alkaloids: Proving Grounds for New Catalytic Transformations
Scheidt, K. A.*; Miller, E. R. Synthesis. 2021, 54, 1217-1230.
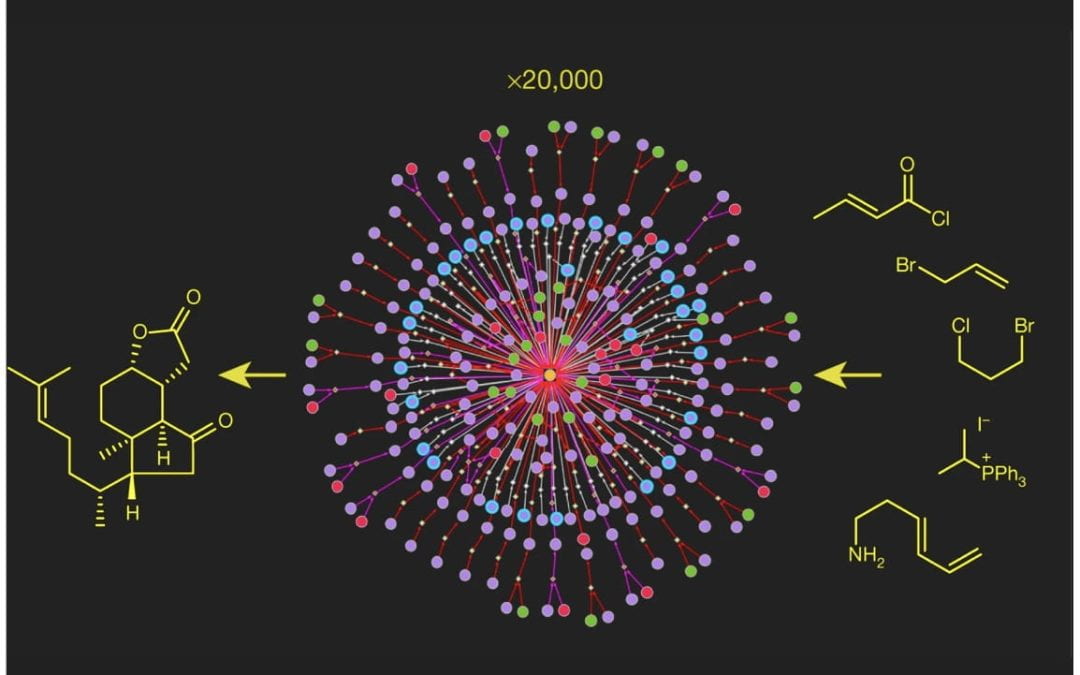
Computational planning of the synthesis of complex natural products
Mikulak-Klucznik, B.; Gołębiowska, P.; Bayly, A. A.; Popik, O.; Klucznik, T.; Szymkuć, S.; Gajewska, E. P.; Dittwald, P.; Staszewska-Krajewska, O.; Beker, W.; Badowski, T.; Scheidt, K. A.; Molga, K.*; Młynarski, J.*; Mrksich, M.*; Grzybowski, B. A.*
Nature 2020, 588, 83-88.
Completed Targets
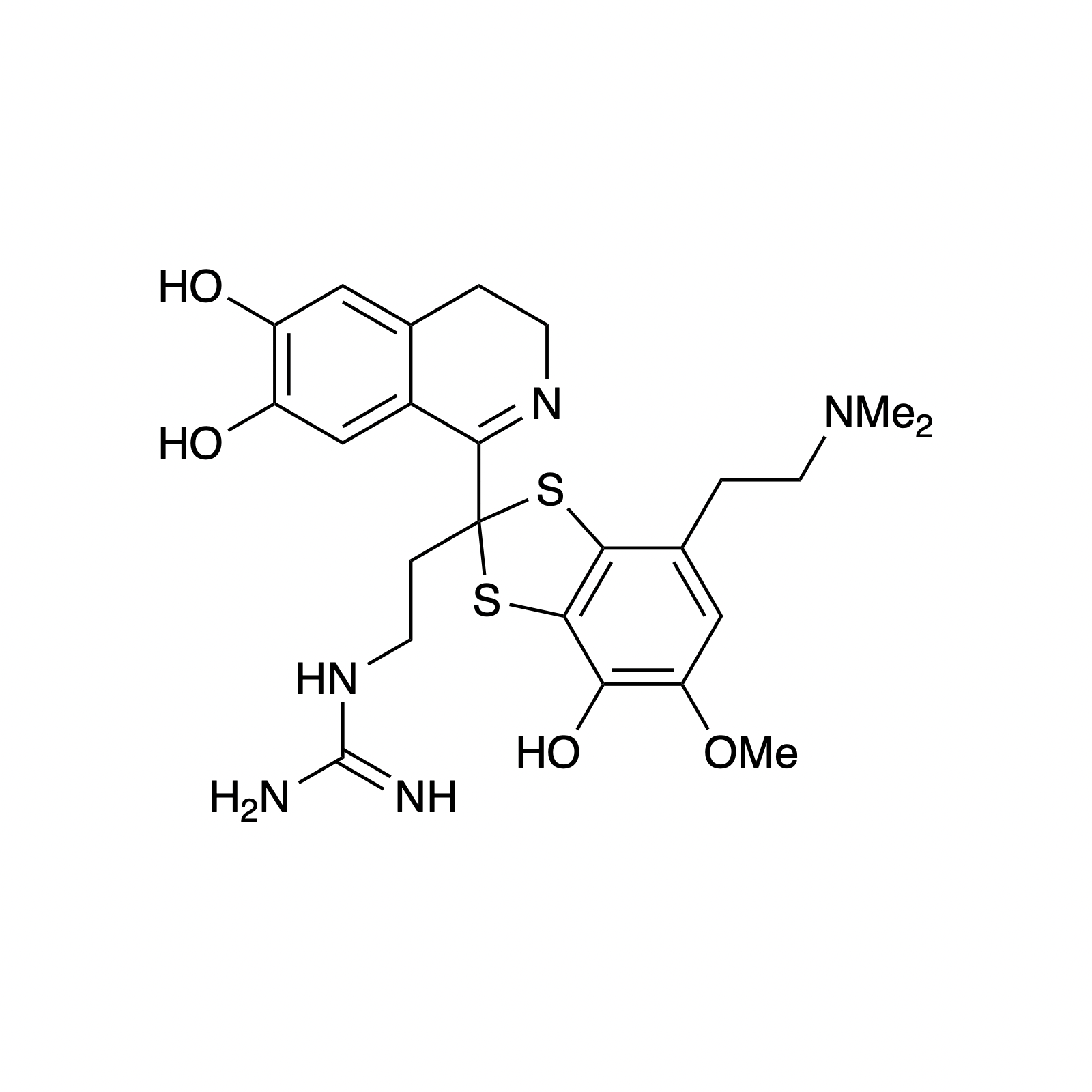
mellpaladine A
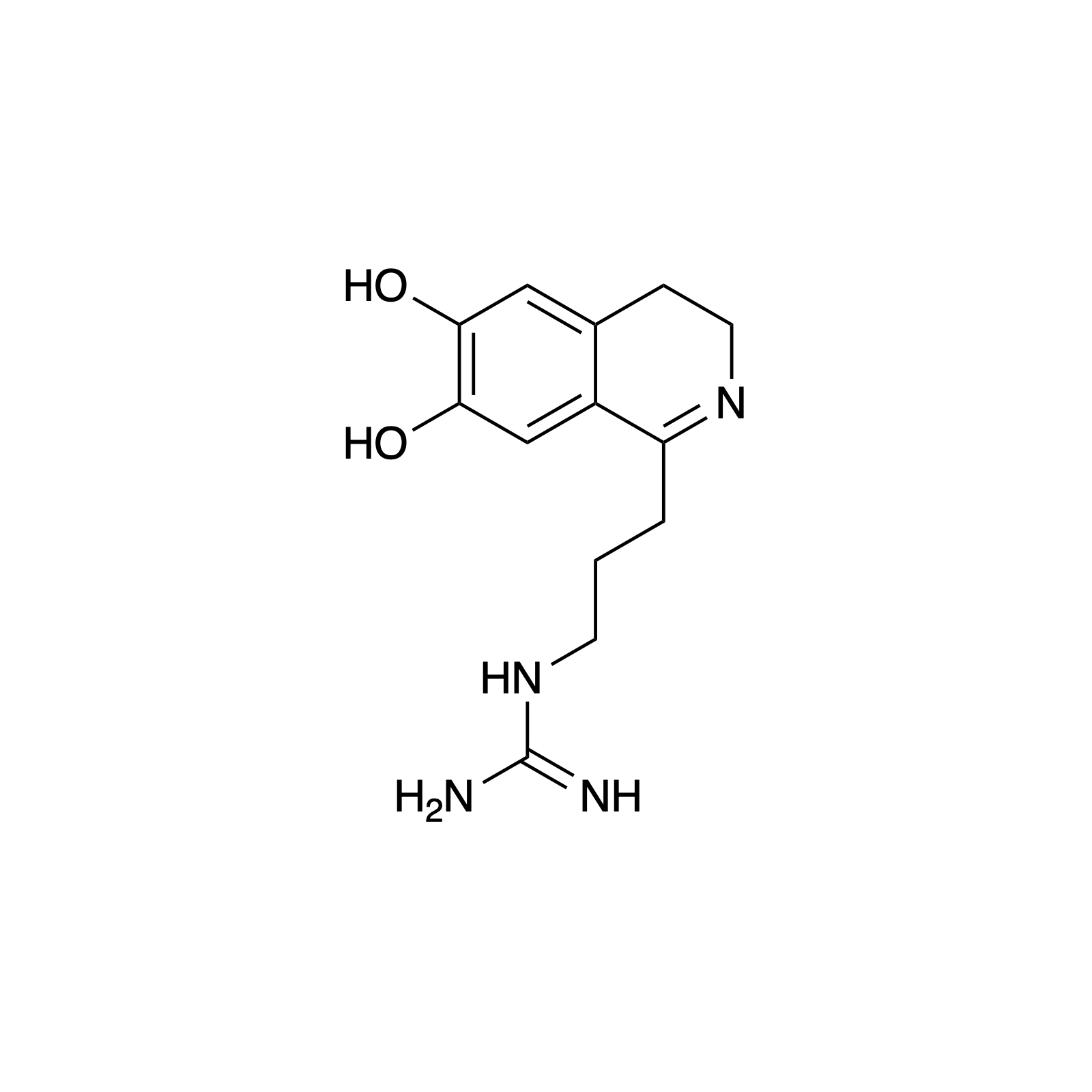
dopargimine
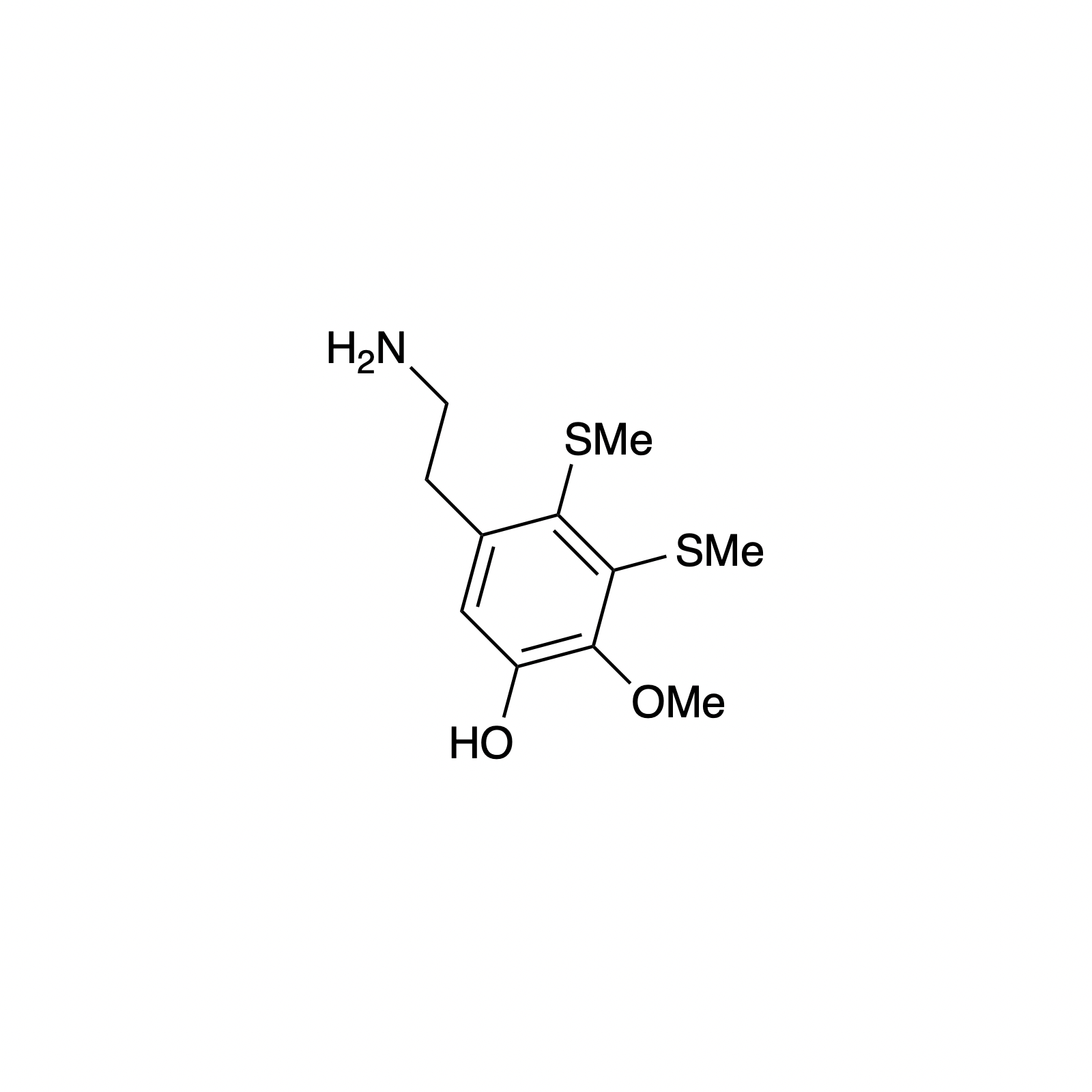
lissoclinotoxin C