
Discovery of DCAF16 Binders for Targeted Protein Degradation
Campos, M. A.; Riha, I. A.; Zhang, C.; Mozes, C.; Scheidt, K. A.*, Zhang, X.*ACS Chemical Biology, 2025, 20, 2, 479–488.

C(sp3)–H Carboxylation via Carbene/Photoredox Cooperative Catalysis
Schull, C. R.; Cao, J.; Mitton-Fry, S. R.; Mrksich, M.; Scheidt, K. A.* ACS Catal. 2025, 15, 1287-1293.

Photochemical phosphorus-enabled scaffold remodeling of carboxylic acids
Peng, Q.; Hwang, M. U.; Rentería-Gómez, A.; Mukherjee, P.; Young, R. M; Qiu, Y.; Wasielewski, M. R.; Gutierrez, O.; Scheidt, K. A.* Science 2024, 385, 1471-1477.

Combined Photocatalysis and Lewis Acid Catalysis Strategy for the Oxa-Pictet–Spengler Reactions of Ethers
Tanaka, N.; Mitton-Fry, S.; Hwang, M. U.; Zhu, J. L.; Scheidt, K. A.* ACS Catalysis 2024, 14, 10, 7949–7955.

Dual NHC/HAT-Promoted Esterification to Access α-Aryl Glycines
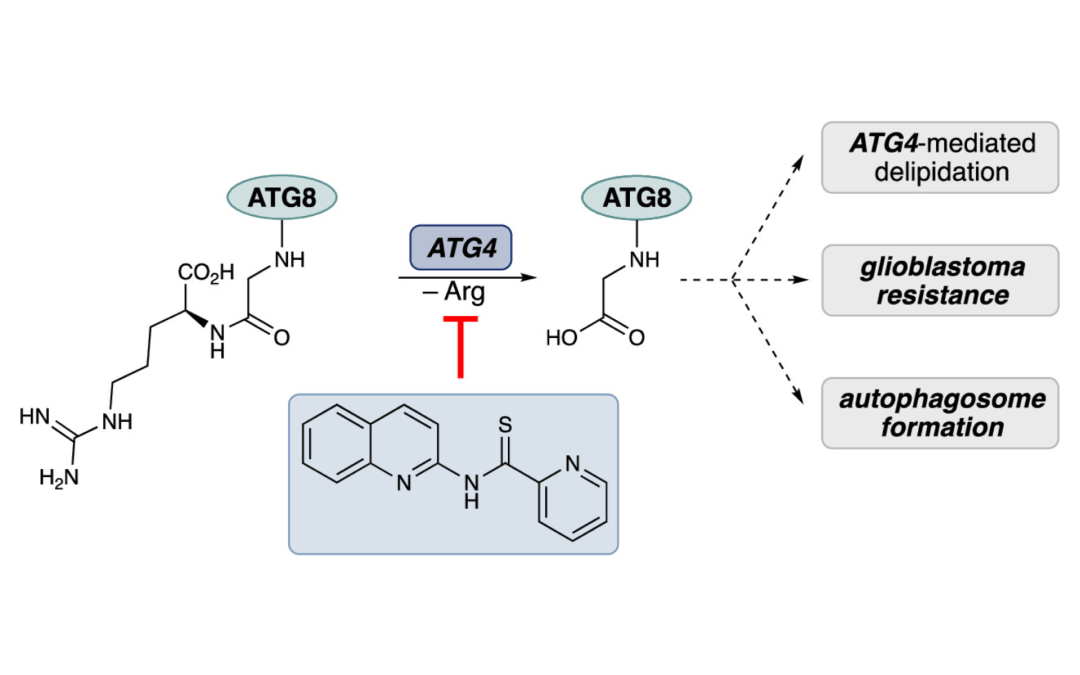
Synthesis and Structural Optimization of ATG4B Inhibitors for the Attenuation of Autophagy in Glioblastoma
Kim. D. R.; Orr, M. J.; Yu, X.; Munshi, H. H.; Wang, A.; Trudeau, C.; Kwong, A. J.; Cheng, S.-Y.; Scheidt, K. A.* ACS Med. Chem. Lett. 2024, 15, 258-264.
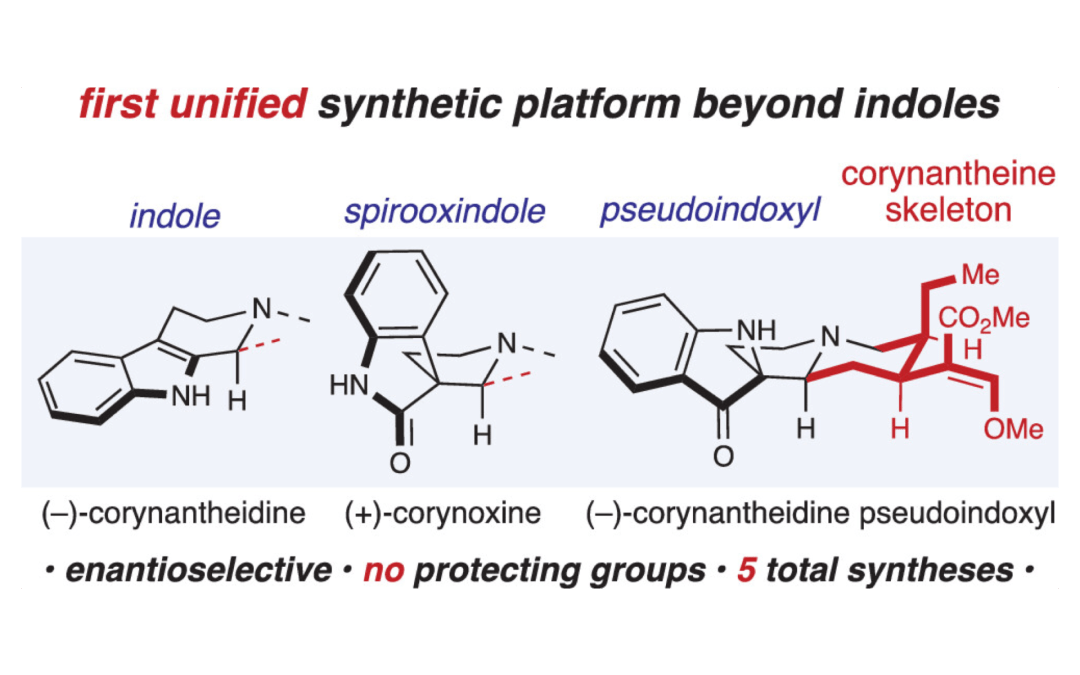
A Platform for the Synthesis of Corynantheine-Type Corynanthe Alkaloids
Nam, Y.; Tam, A. T.; Miller, E. R.; Scheidt, K. A.* J. Am. Chem. Soc. 2024, 146, 118-124.
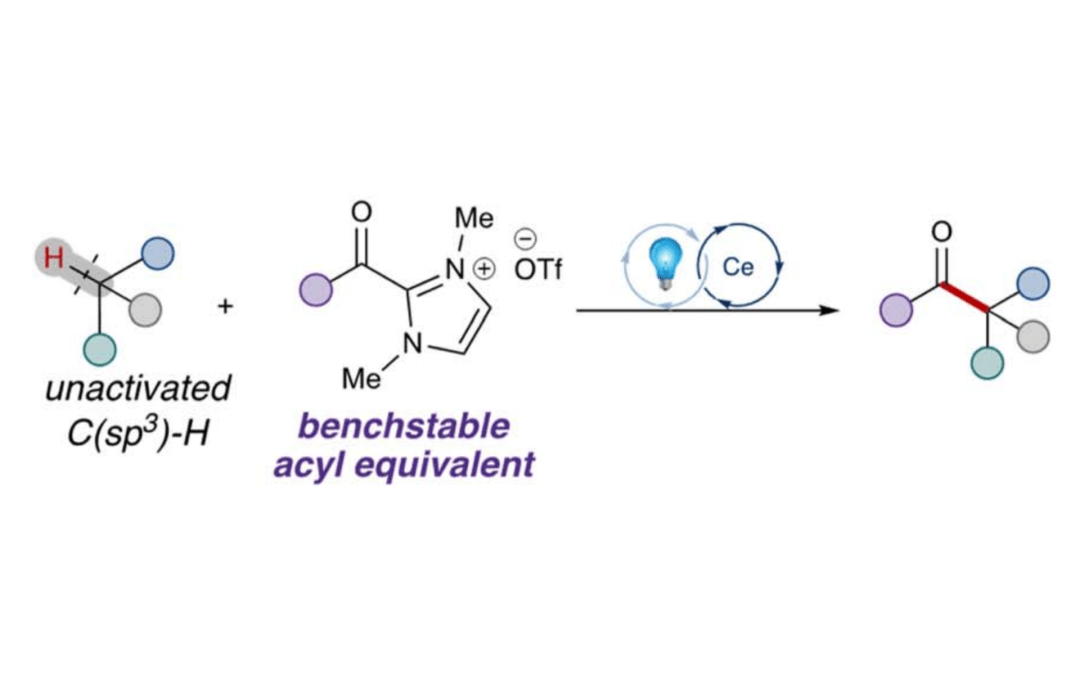
Photoinduced cerium-catalyzed C–H acylation of unactivated alkanes
Cao, J,; Zhu, J. L.; Scheidt, K. A.* Chem. Sci. 2023, 15, 154-159.
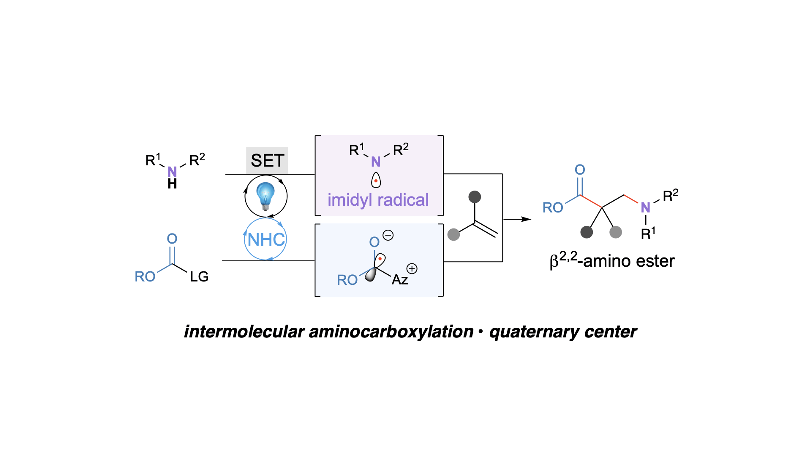
Cooperative Carbene Photocatalysis for β-Amino Ester Synthesis
Tanaka, N.; Zhu, J. L.; Valencia, O. L.; Schull, C. R.; Scheidt, K. A.* J. Am. Chem. Soc. 2023, 145, 24486-24492.
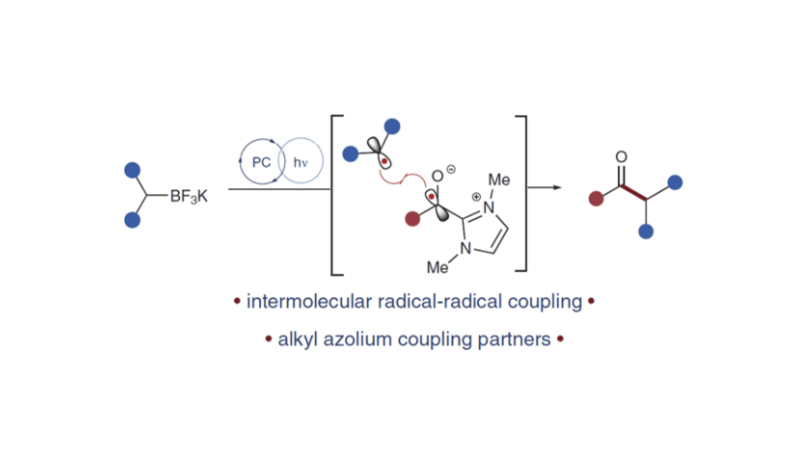
Photoredox-Catalyzed Radical–Radical Coupling of Potassium Trifluoroborates with Acyl Azoliums
Rourke, M. J.; McGill, M. J.; Yang, D.; Farnam, E.J.; Zhu, J.L.; Scheidt, K. A.* Synlett. 2023, 34, 2175-2180.
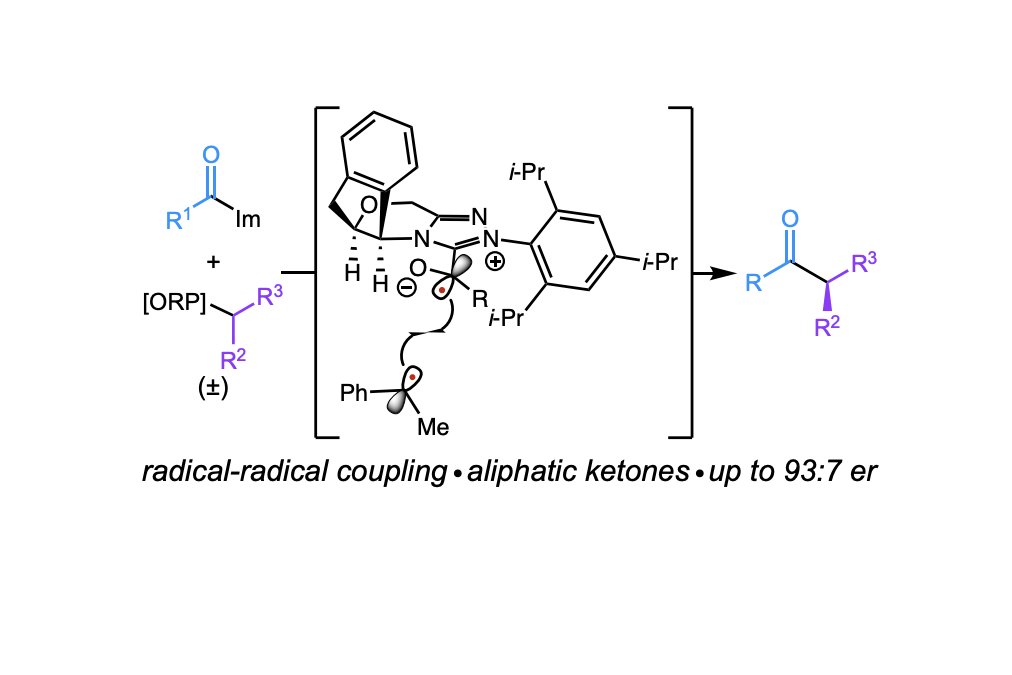
Light-Driven Enantioselective Carbene-Catalyzed Radical-Radical Coupling
Byun, S.; Hwang, M.; Wise, H.; Bay, A.; Cheong, P.; Scheidt, K. A.* Angew. Chem. Int. Ed. 2023, 62, e2023128.
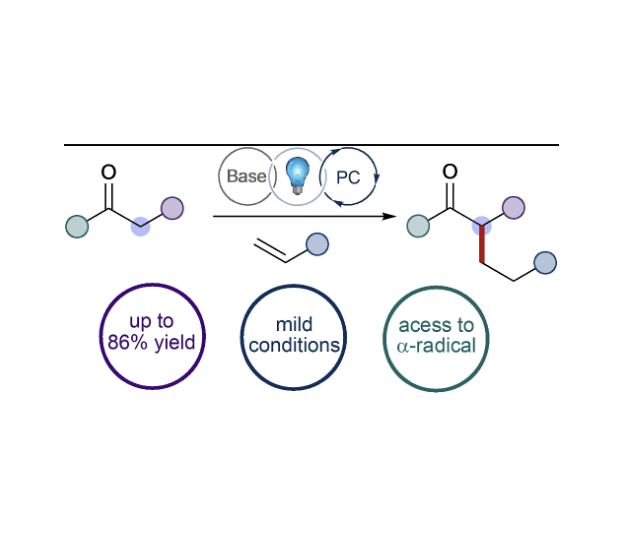
Photocatalyzed Direct α-Alkylation of Esters Using Styrenes
Wang, P.; Bay, A. V.; Farnam, E. J.; Scheidt, K. A.* Adv. Synth. Catal. 2023, 365, 2361-2366.
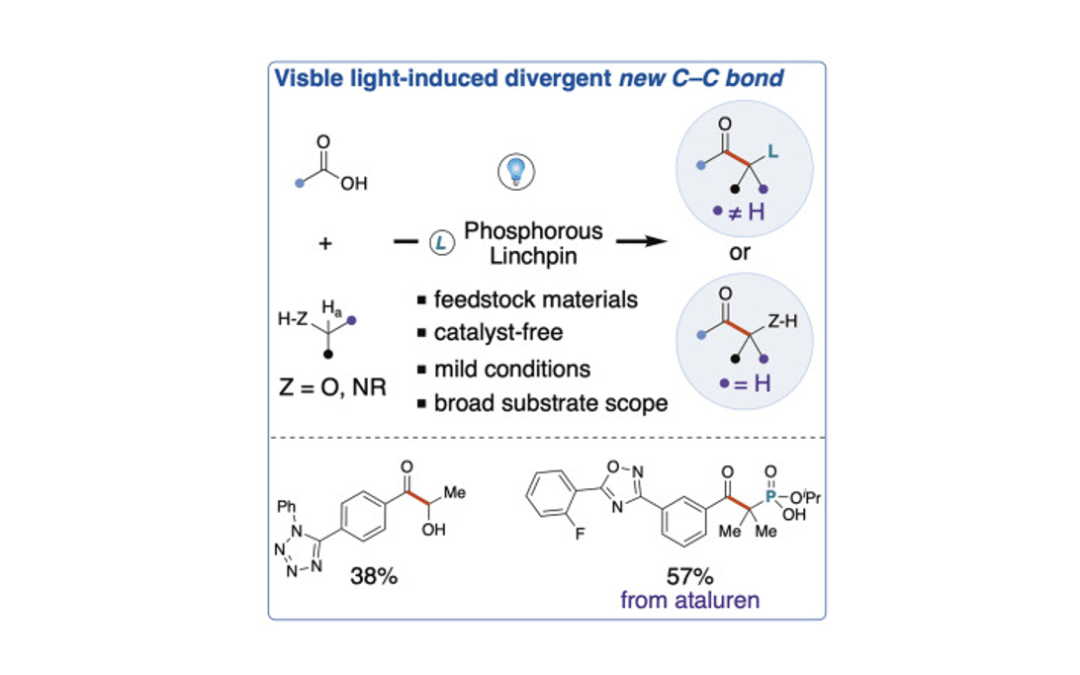
Visible light-induced coupling of carboxylic acids with alcohols/amines via a phosphorous linchpin strategy
Peng, Q.; Gogoi, A. R.; Renteria-Gomez, A.; Gutierrez, O.; Scheidt, K. A.* Chem 2023, 9, 1983-1993.
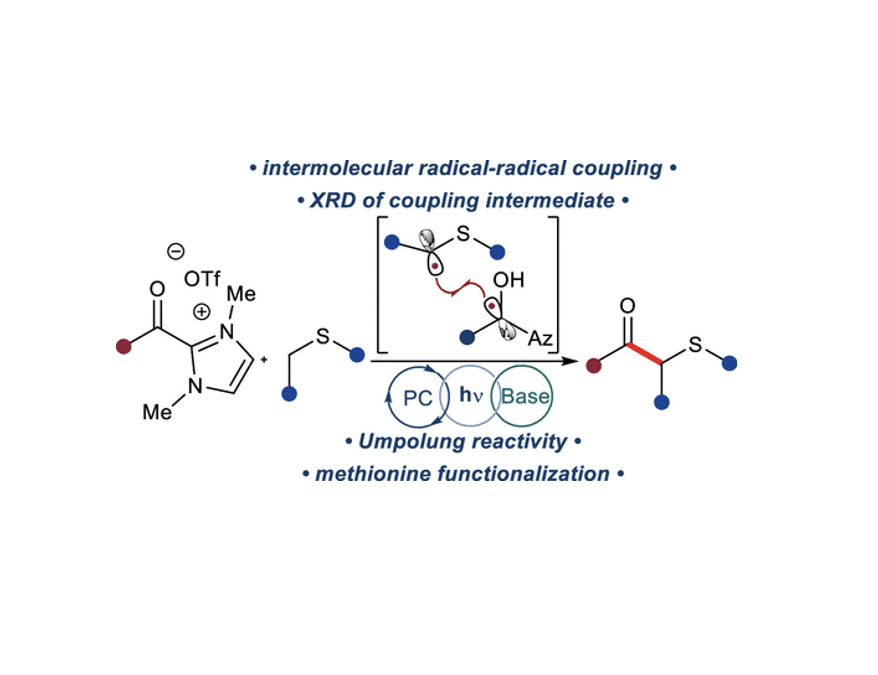
Acyl Azolium–Photoredox-Enabled Synthesis of β-Keto Sulfides
Rourke, M.J.; Wang, C.T.; Schull, C.R.; Scheidt, K. A.* ACS Catal. 2023, 13, 7987-7994.
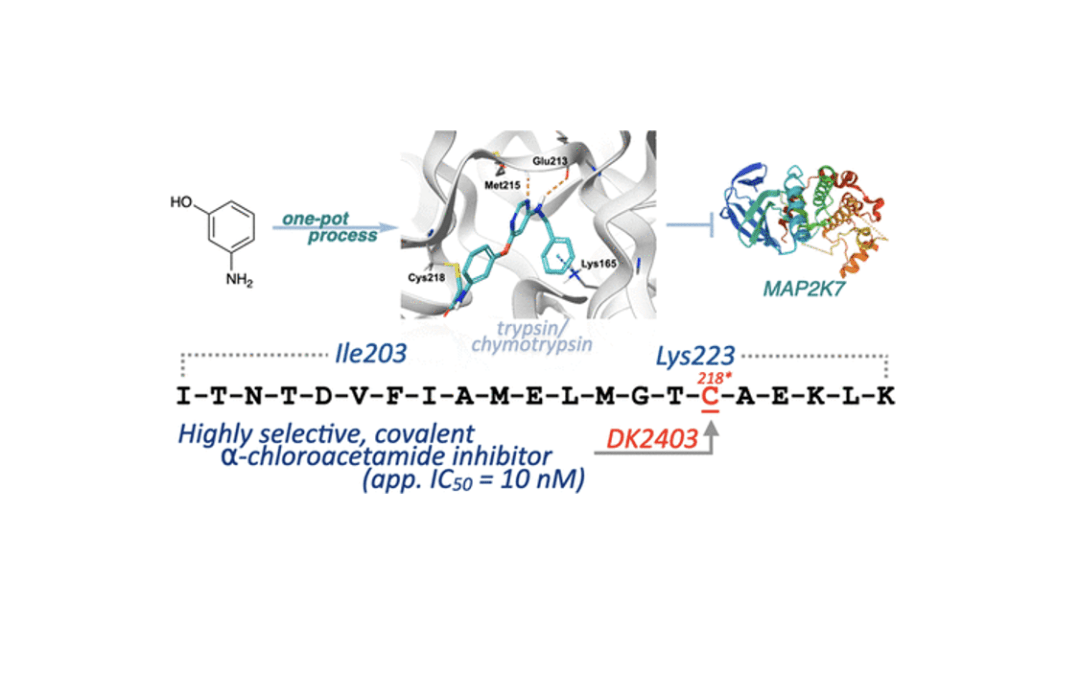
Rational Design of Highly Potent and Selective Covalent MAP2K7 Inhibitors
Kim, D. R; Orr, M. J.; Kwong, A. J.; Deibler, K. K.; Munshi, H. H.; Bridges, C. S; Chen, T. J.; Zhang, X.; Lacorazza, H. D.; Scheidt, K. A.* ACS Med. Chem. Lett. 2023, 14, 606-613.
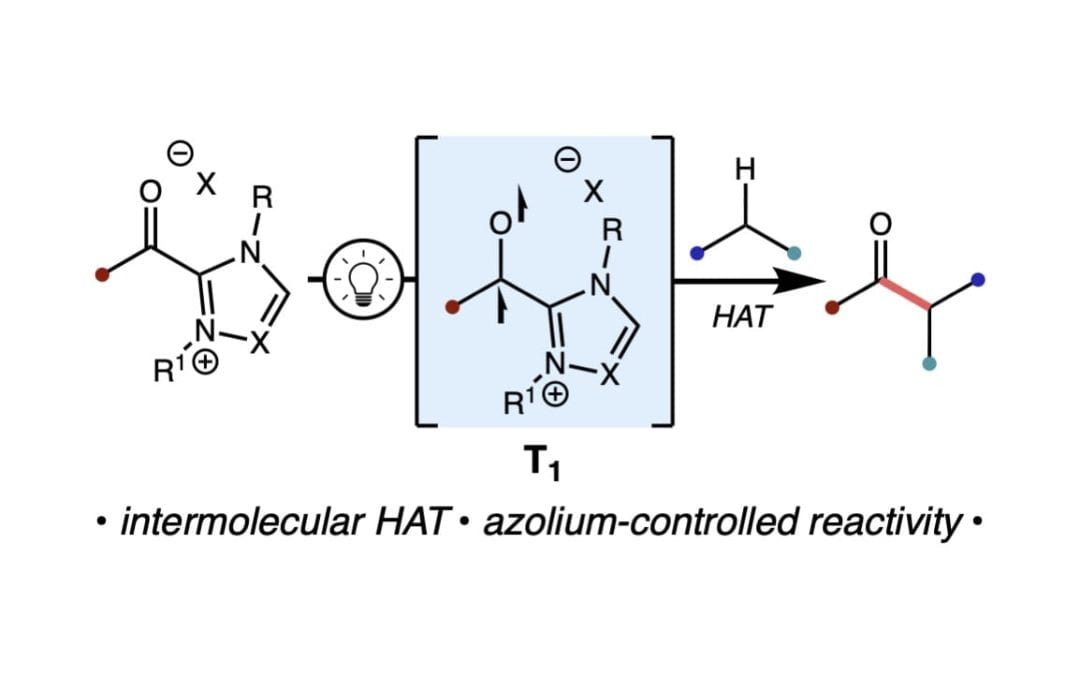
Photoinduced Acylations Via Azolium-Promoted Intermolecular Hydrogen Atom Transfer
Zhu, J. L.; Schull, C. R.; Tam, A. T.; Renteria-Gomez, A.; Gogoi, A. R.; Gutierrez, O.; Scheidt, K. A.* J. Am. Chem. Soc. 2023, 145, 1535-1541.
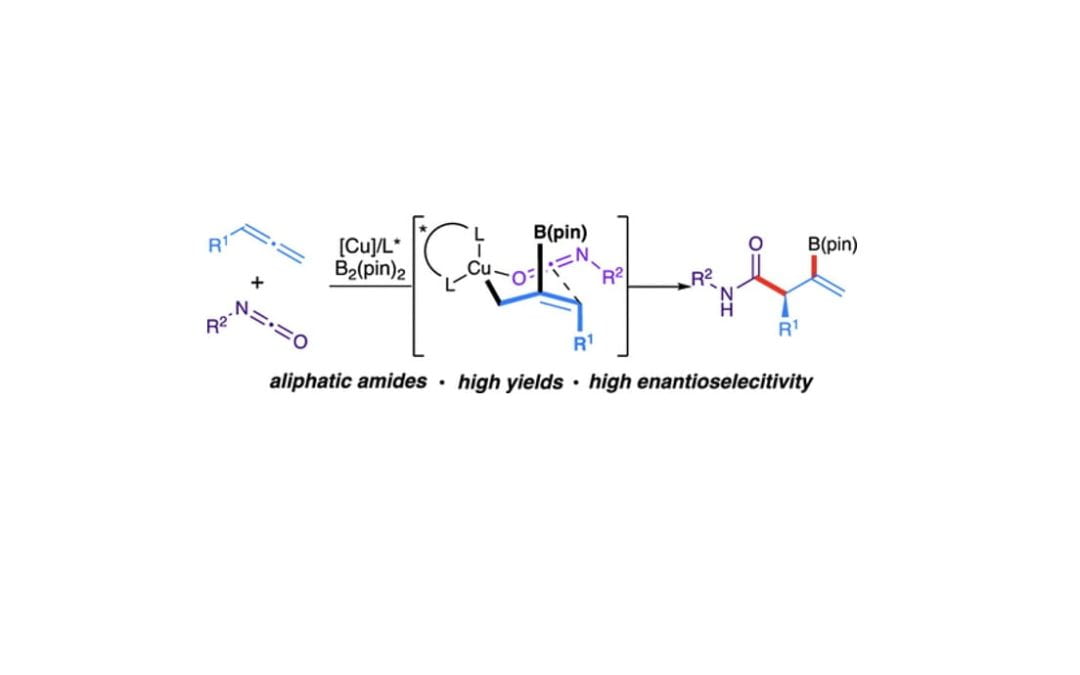
Enantioselective Copper-Catalyzed Borylative Amidation of Allenes
Byun, S.; Farah, A.O.; Wise, H.R.; Katchmar, A.; Cheong, P.H.-Y; Scheidt, K.A* J. Am. Chem. Soc. 2022, 144, 22850-22857.
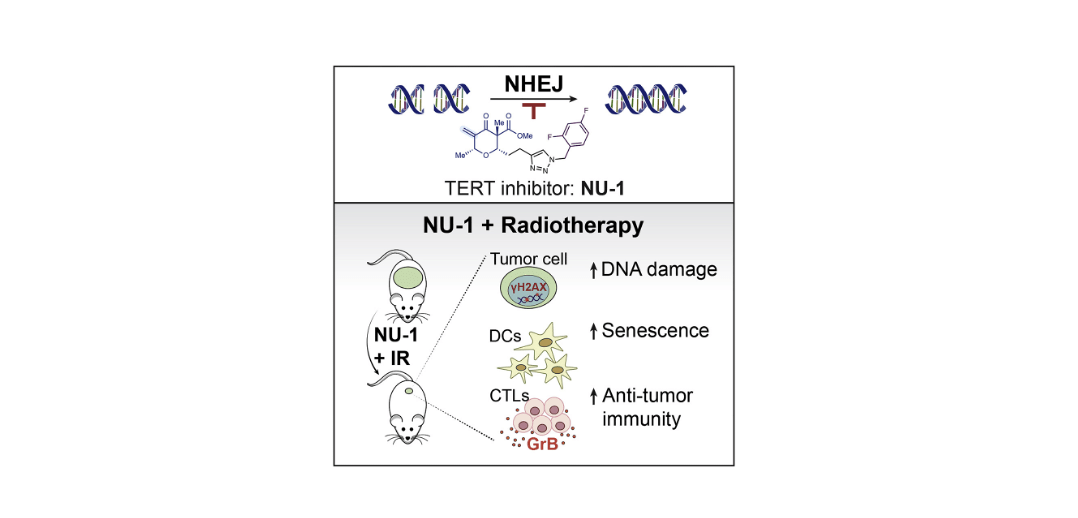
Targeting telomerase reverse transcriptase with the covalent inhibitor NU-1 confers immunogenic radiation sensitization
Liu, Y.; Betori, R. C.; Pagacz, J.; Frost, G. B.; Efimova, E. V.; Wu, D.; Wolfgeher, D. J.; Bryan, T. M.; Cohen, S. B.; Scheidt, K. A.; Kron, S. J.* Cell Chem. Biol. 2022, 29, 1517-1531.
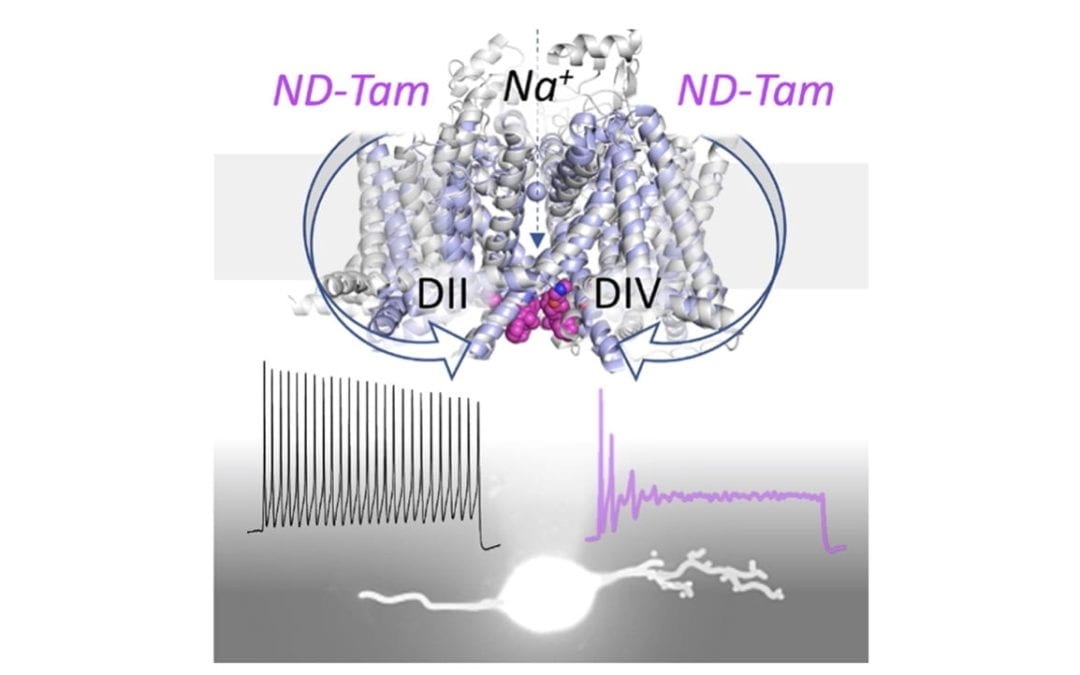
Targeting the tamoxifen receptor within sodium channels to block osteoarthritic pain
McCollum, M. M.; Larmore, M.; Ishihara, S.; Ng, L. C. T.; Kimura, L. F.; Guadarrama, E.; Ta, M. C.; Vien, T. N.; Frost, G. B.; Scheidt, K. A.; Miller, R. E.; DeCaen, P. G.* Cell Rep. 2022, 40, 111248.
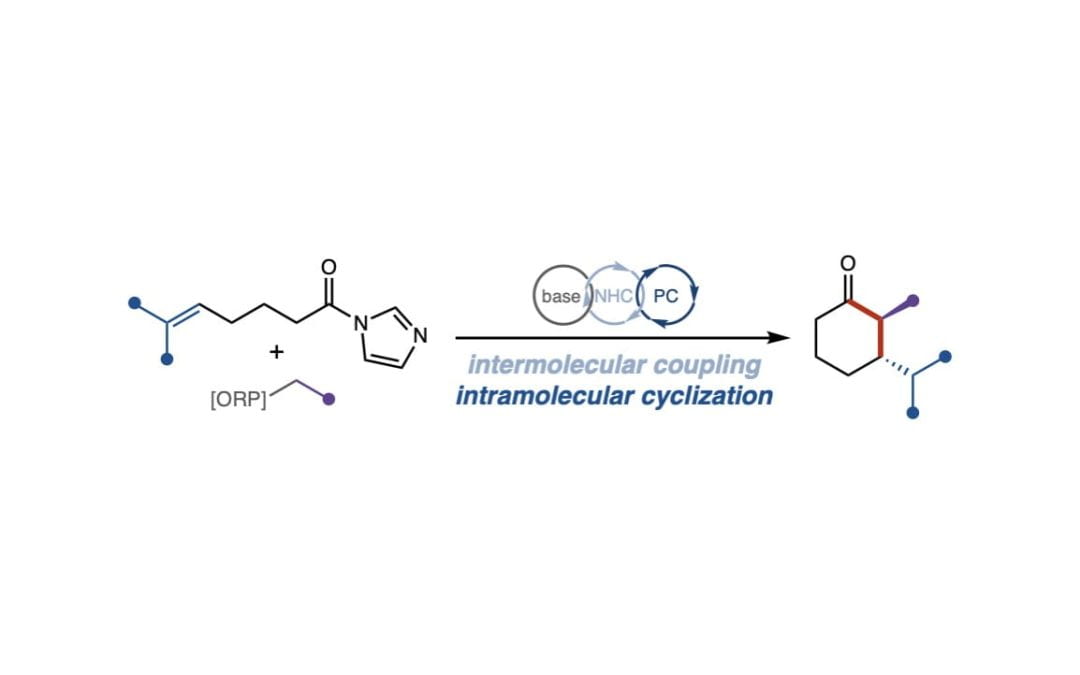
Synthesis of Cyclohexanones by a Tandem Photocatalyzed Annulation
Bay, A. V.; Farnam, E. J.; Scheidt, K. A.* J. Am. Chem. Soc. 2022, 144, 7030-7037.
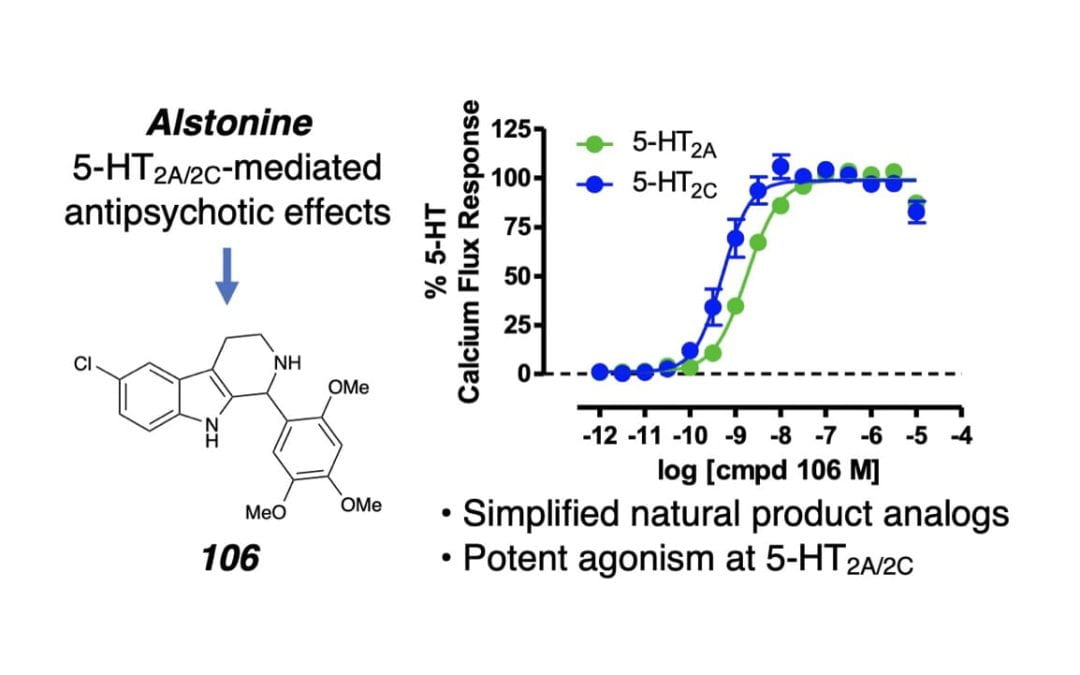
Discovery of Highly Potent Serotonin 5-HT2 Receptor Agonists Inspired by Heteroyohimbine Natural Products
Orr, M. J.; Cao, A. B.; Wang, C. T.; Gaisin, A.; Csakai, A.; Friswold, A. P.; Meltzer, H. Y.; McCorvy, J. D.*; Scheidt, K. A.* ACS Med. Chem. Lett. 2022, 13, 648-657.
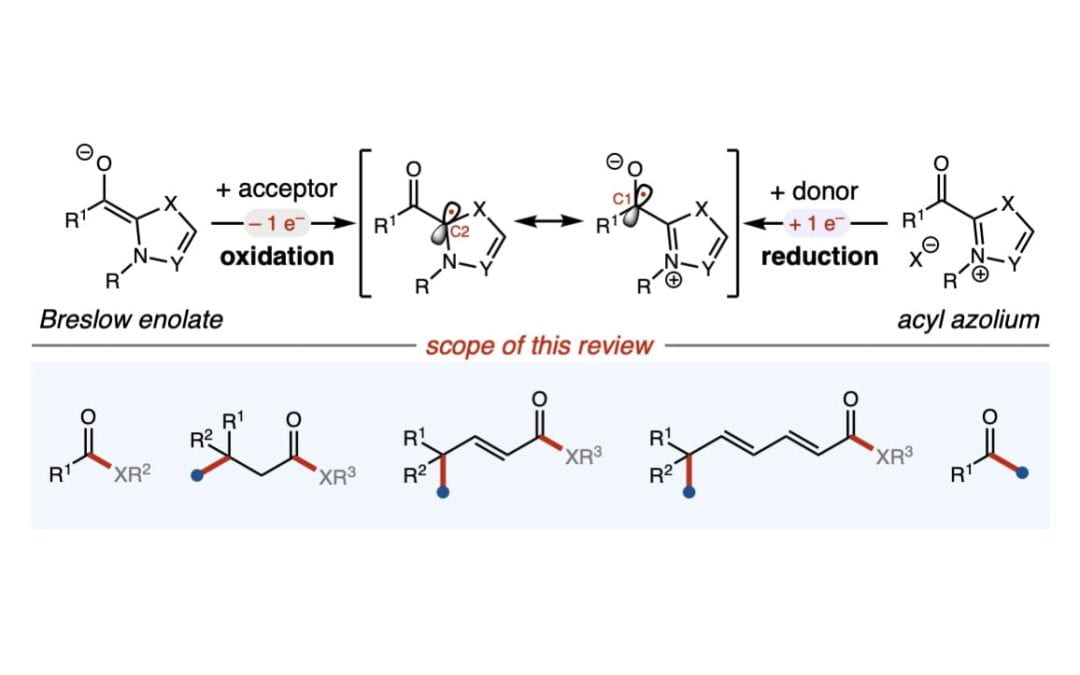
Single-electron carbene catalysis in redox processes
Bay, A. V.; Scheidt, K. A.* Trends Chem. 2022, 4, 277-290.
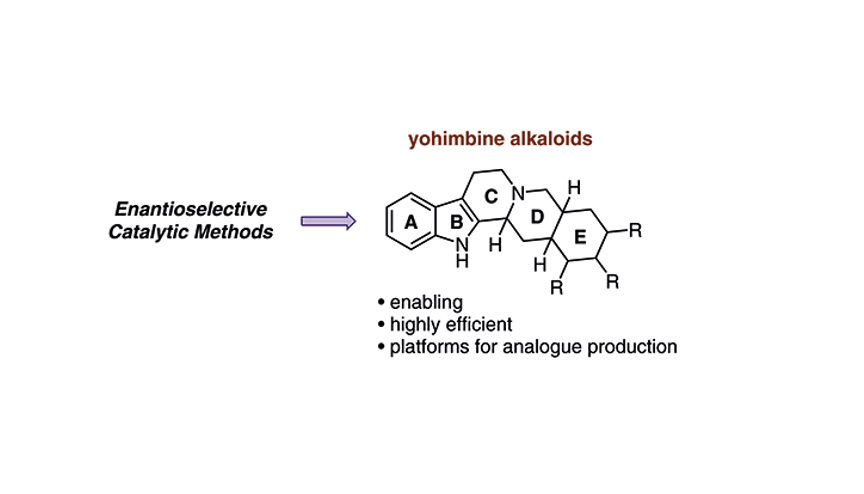
Enantioselective Syntheses of Yohimbine Alkaloids: Proving Grounds for New Catalytic Transformations
Scheidt, K. A.*; Miller, E. R. Synthesis. 2021, 54, 1217-1230.
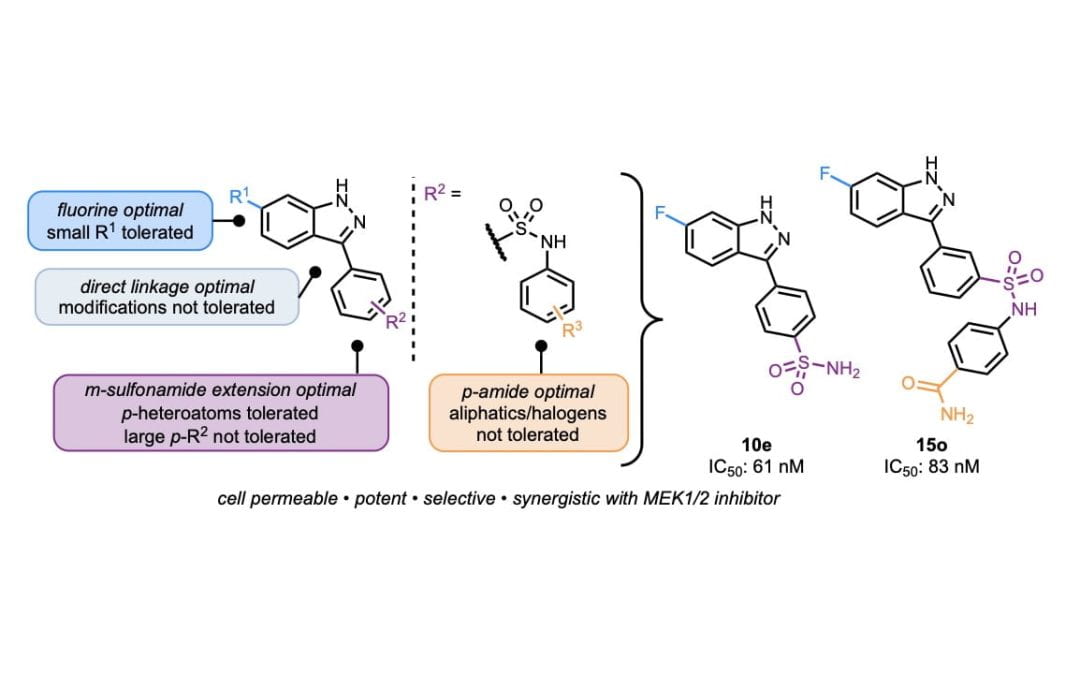
Rational Design, Optimization, and Biological Evaluation of Novel MEK4 Inhibitors against Pancreatic Adenocarcinoma
Kwong, A. J.; Pham, T. N. D.; Oelschlager, H. E.; Munshi, H. G.*; Scheidt, K. A.* ACS Med. Chem. Lett. 2021, 12, 1559-1567.
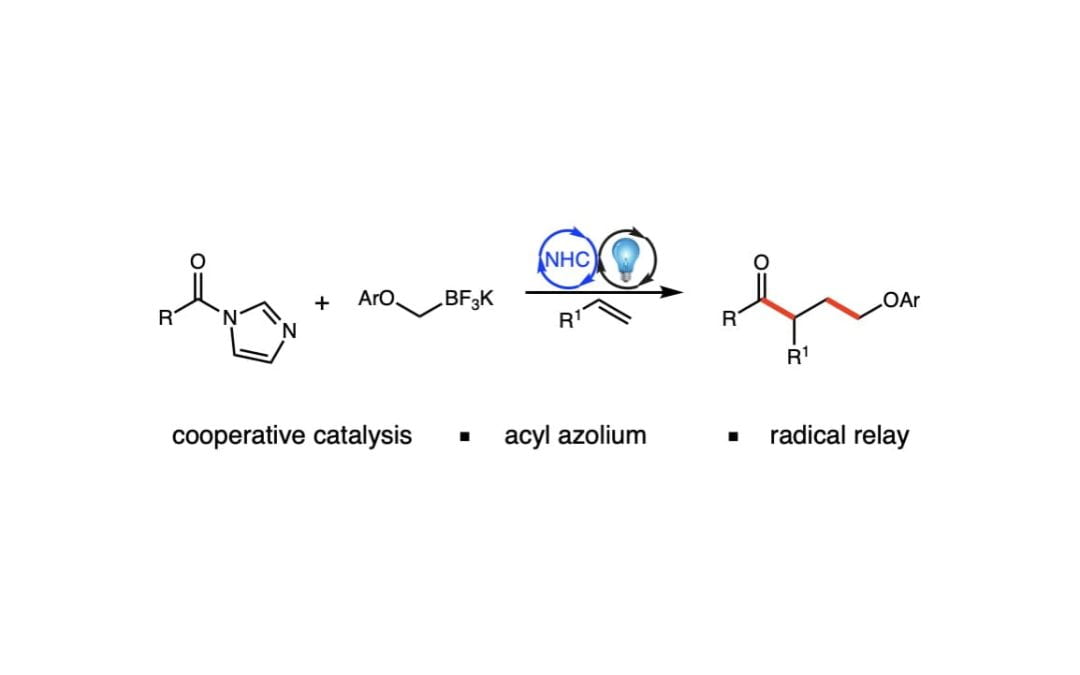
Combined Photoredox and Carbene Catalysis for the Synthesis of γ-Aryloxy Ketones
Wang, P.; Fitzpatrick, K. P.; Scheidt, K. A.* Adv. Synth. Catal. 2021, 364, 518-524.
Advanced Synthesis and Catalysis Top 10 Most Cited 2022-2023.
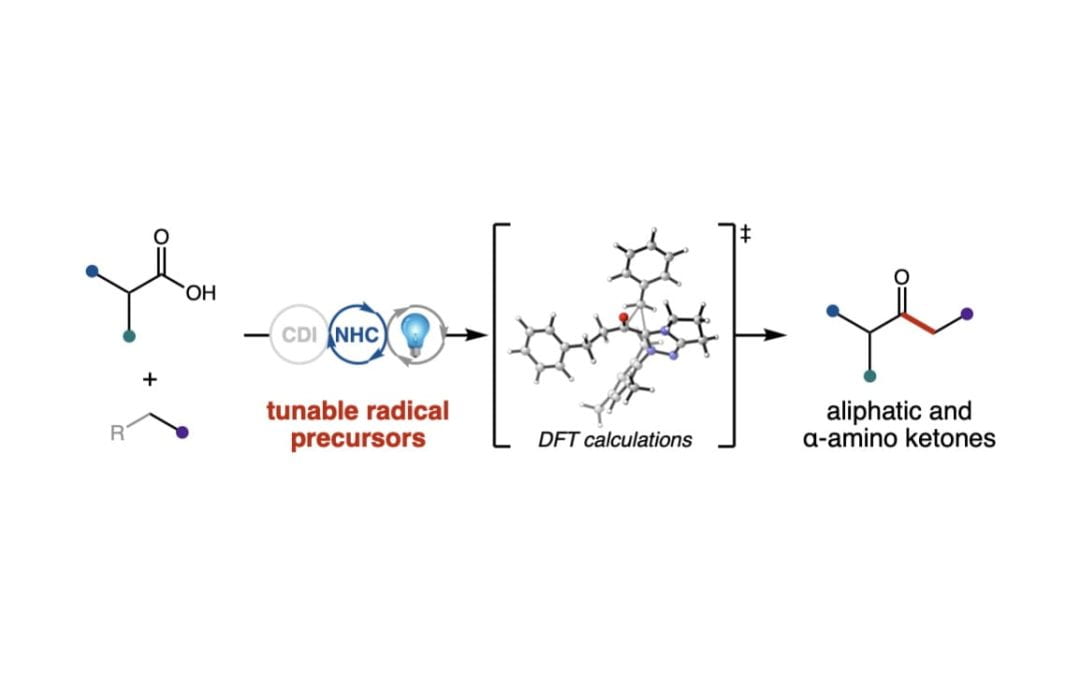
Light-Driven Carbene Catalysis for the Synthesis of Aliphatic and α-Amino Ketones
Bay, A. V.; Fitzpatrick, K. P.; González-Montiel, G. A.; Farah, A. O.; Cheong, P. H.-Y.*; Scheidt, K. A.* Angew. Chem. Int. Ed. 2021, 60, 17925-17931.
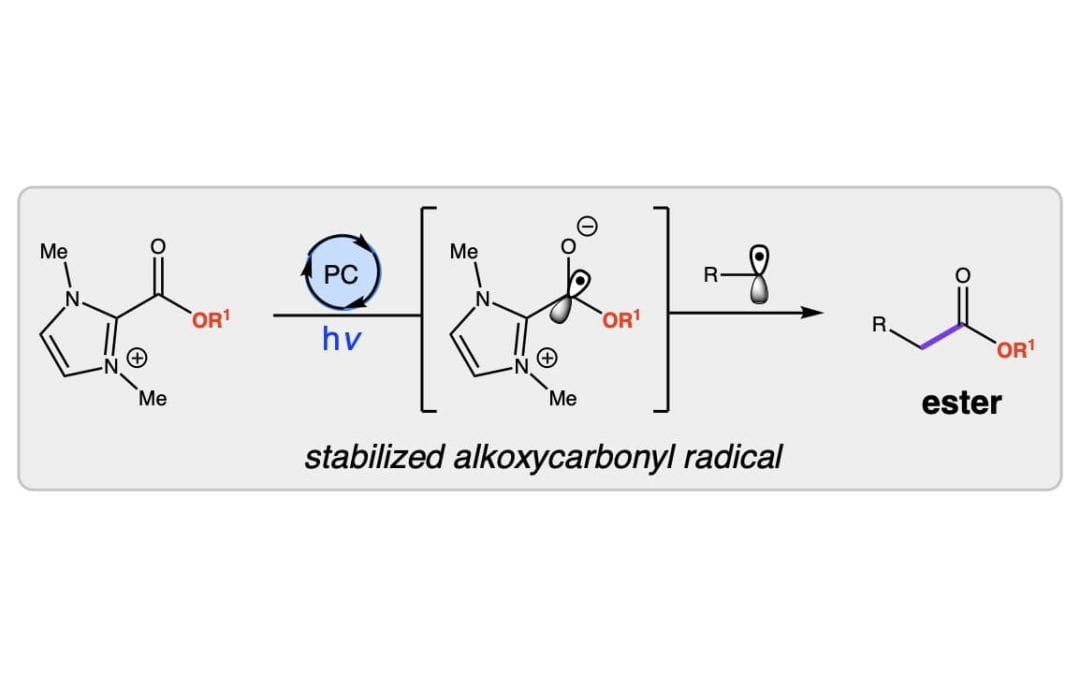
Photocatalytic acyl azolium-promoted alkoxycarbonylation of trifluoroborates
Zhu, J. L.; Scheidt, K. A.* Tetrahedron 2021, 92, 132288.
Featured in: 2021 Editor’s Choice Collection
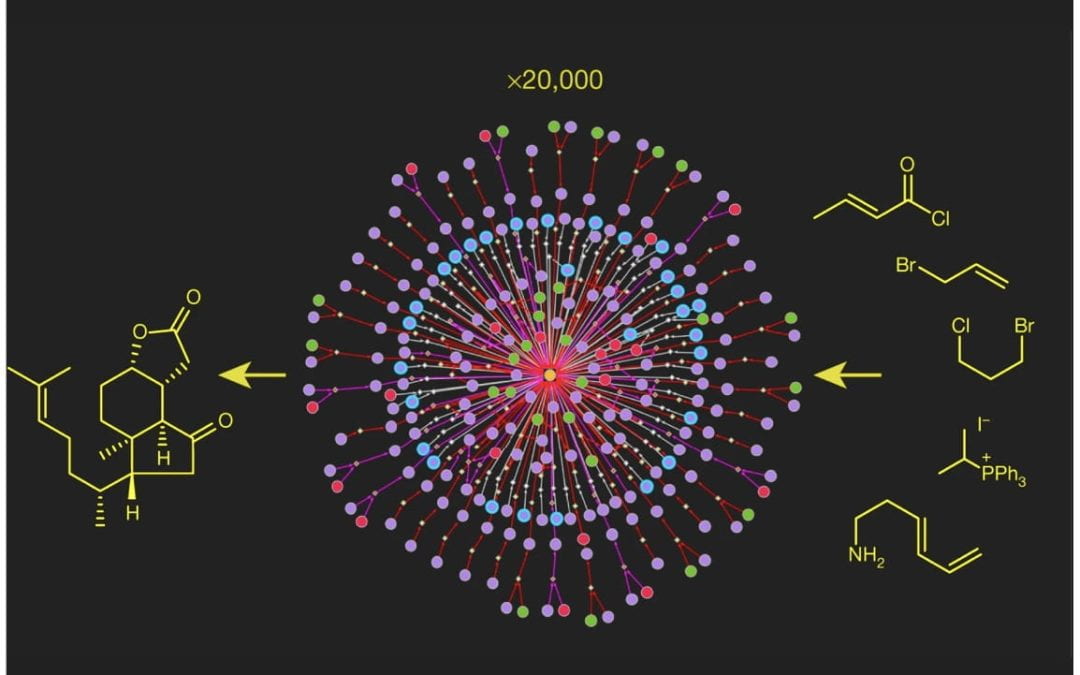
Computational planning of the synthesis of complex natural products
Mikulak-Klucznik, B.; Gołębiowska, P.; Bayly, A. A.; Popik, O.; Klucznik, T.; Szymkuć, S.; Gajewska, E. P.; Dittwald, P.; Staszewska-Krajewska, O.; Beker, W.; Badowski, T.; Scheidt, K. A.; Molga, K.*; Młynarski, J.*; Mrksich, M.*; Grzybowski, B. A.*
Nature 2020, 588, 83-88.
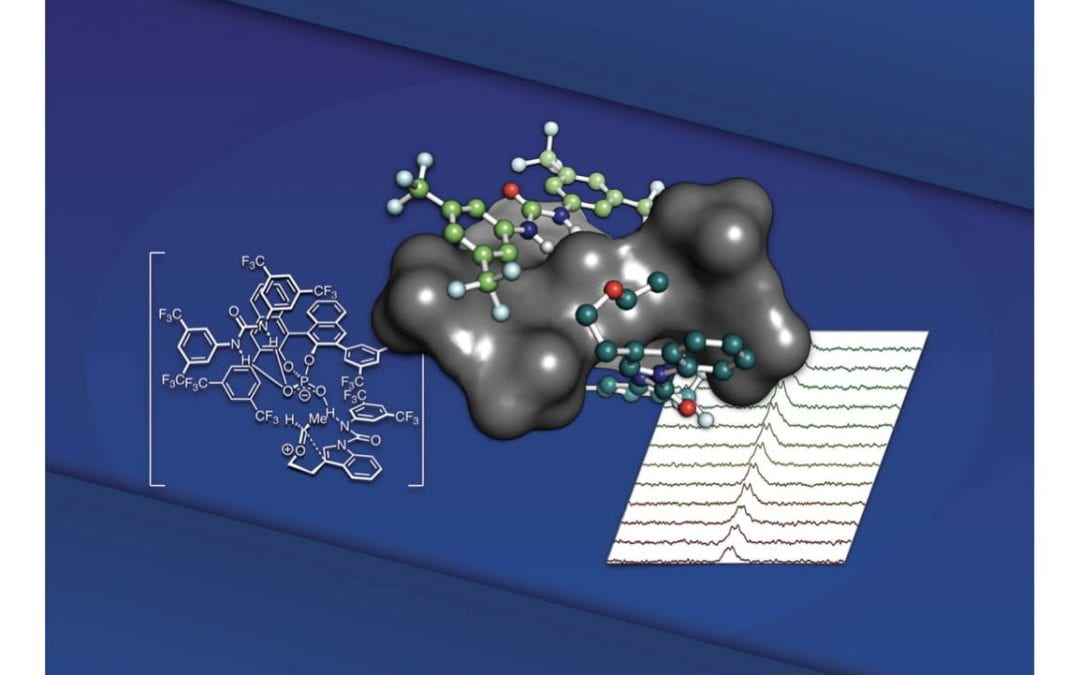
Mechanism and Origins of Selectivity in the Enantioselective oxa-Pictet-Spengler Reaction: A Cooperative Catalytic Complex from a Hydrogen Bond Donor and Chiral Phosphoric Acid
Maskeri, M. A.‡; Brueckner, A. C.‡; Feoktistova, T.; O’Connor, M. J.; Walden, D. M.; Cheong P. H.-Y.*; Scheidt, K. A.*
Chem. Sci. 2020, 11, 8736-8743.
‡Authors contributed equally to this work
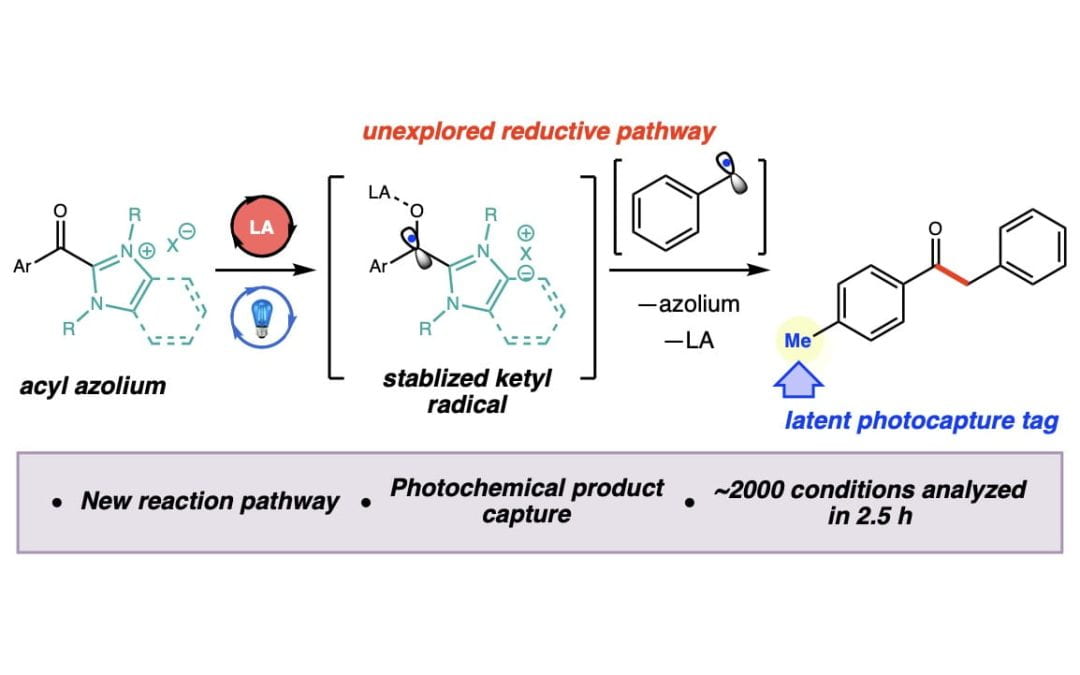
High-throughput photocapture approach for reaction discovery
Bayly, A. A.; McDonald, B. R.; Mrksich, M.*; Scheidt, K. A.*
Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 13261-13266.
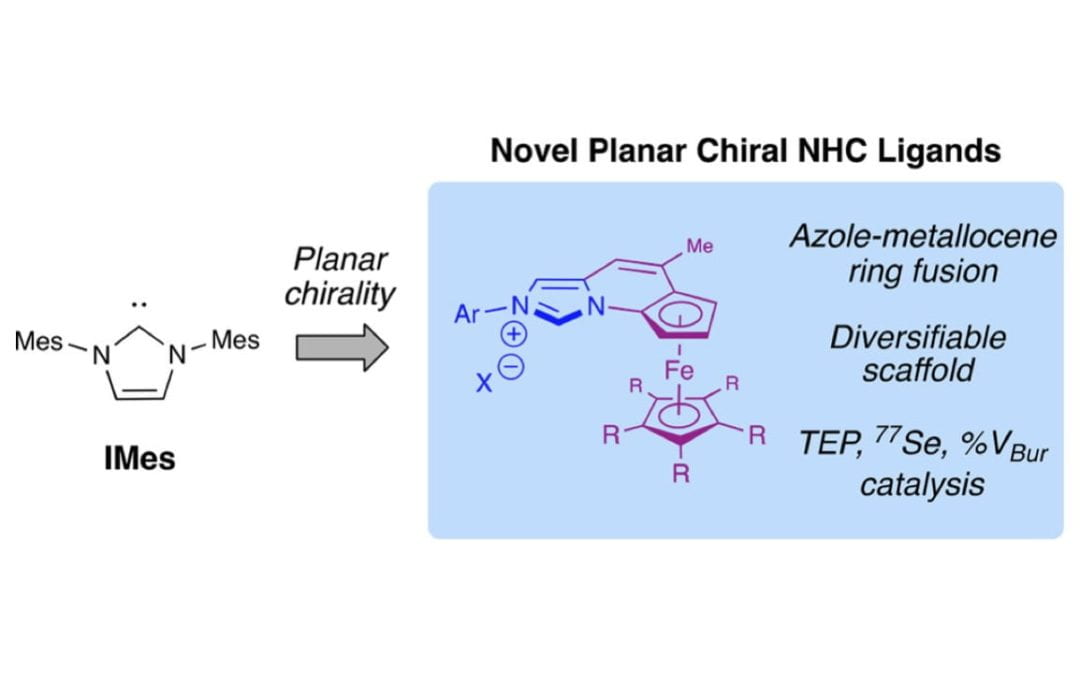
Development of Ferrocene-Based Planar Chiral Imidazopyridinium Salts for Catalysis
Fitzpatrick, K. P.; Schwamb, C. B.; Check, C. T.; Jang, K.-P.; Barsoum, D. N.; Scheidt, K. A.*
Organometallics 2020, 39, 2705-2712.
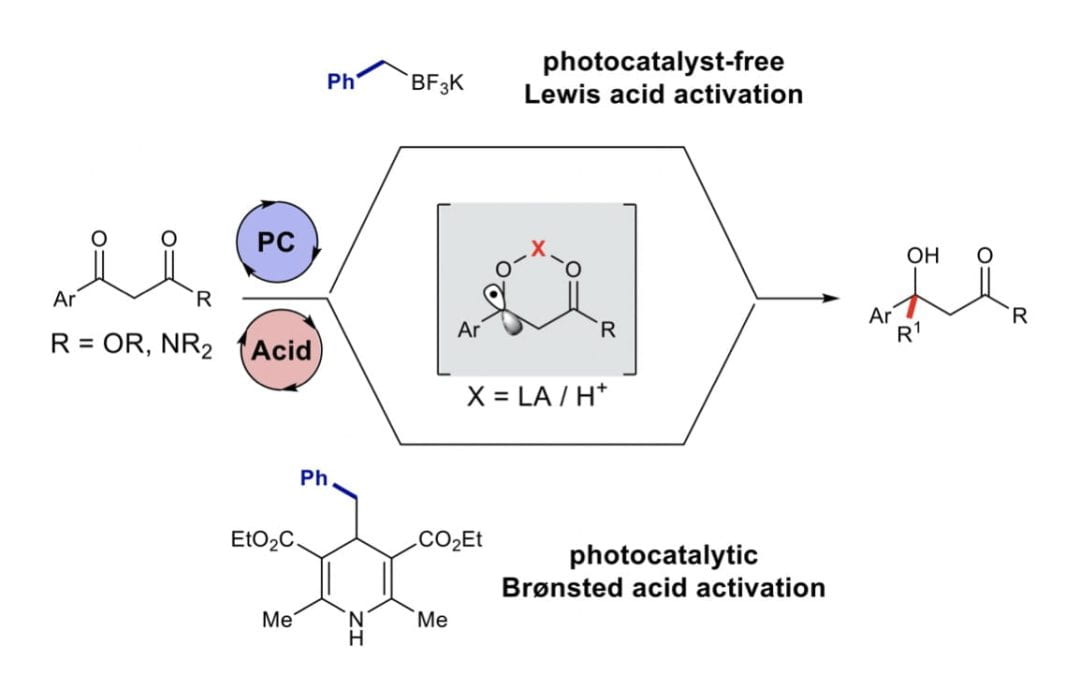
Radical coupling of β-keto esters and amides promoted by Brønsted/Lewis acids
Zhu, J. L.; Laws, S. M.; Rourke, M. J.; Scheidt, K. A.* Green Synth. Catal. 2020, 1, 70-74.
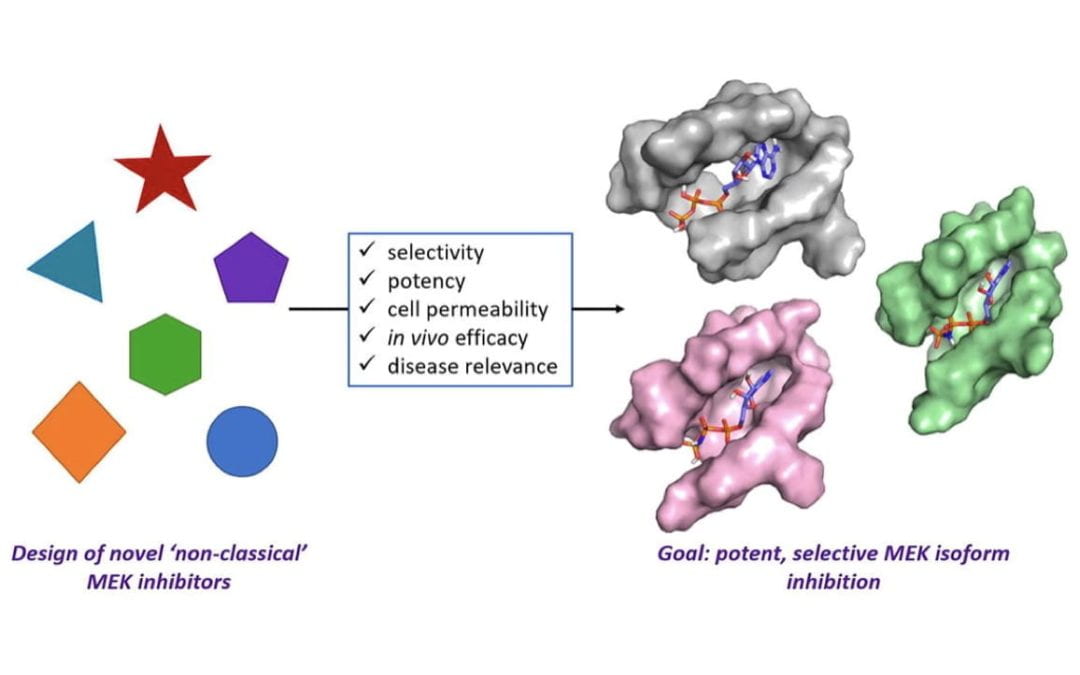
Non-classical MEKs: A review of MEK3-7 inhibitors
Kwong, A. J.; Scheidt, K. A.*
Bioorg. Med. Chem. Lett. 2020, 30, 127203.
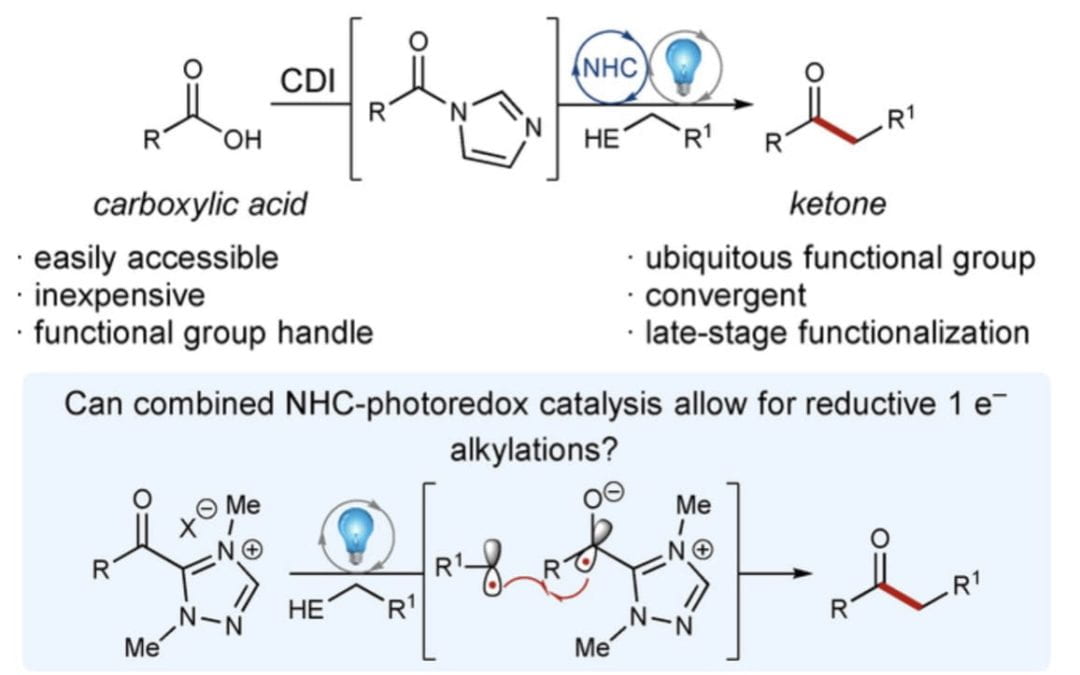
Combined Photoredox and Carbene Catalysis for the Synthesis of Ketones from Carboxylic Acids
Bay, A. V.‡; Fitzpatrick, K. P.‡; Betori, R. C.; Scheidt, K. A.*
Angew. Chem. Int. Ed. 2020, 59, 9143-9148.
‡Authors contributed equally to this work
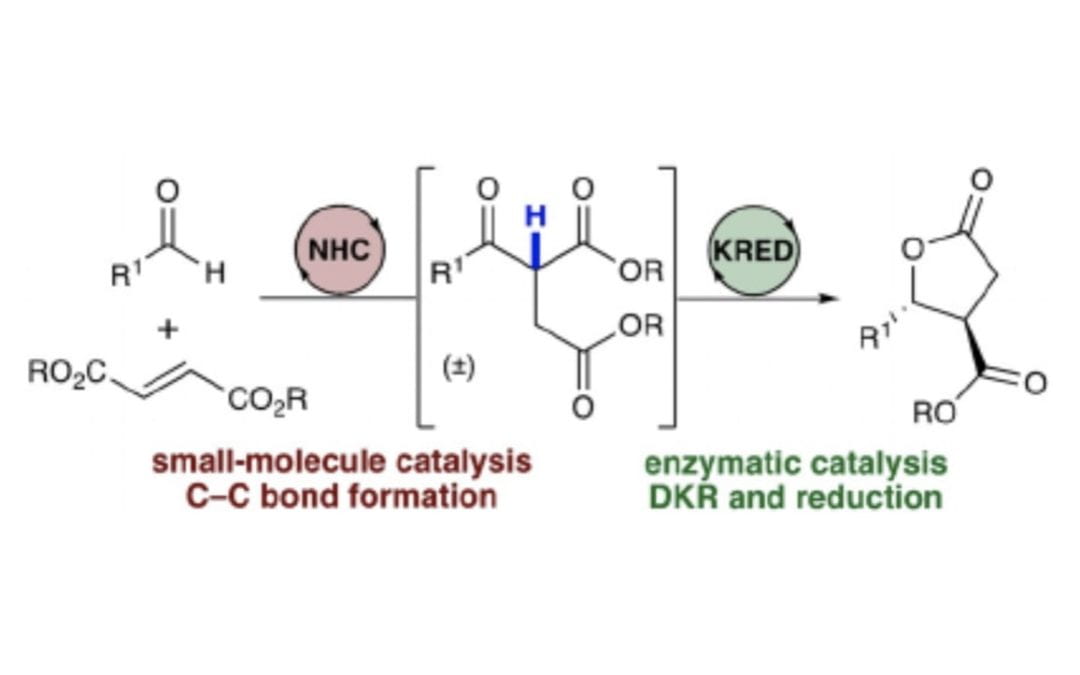
A Sequential Umpolung/Enzymatic Dynamic Kinetic Resolution Strategy for the Synthesis of γ-Lactones
Maskeri, M. A.; Schrader, M. L.; Scheidt, K. A.*
Chem. Eur. J. 2020, 26, 5794-5798.
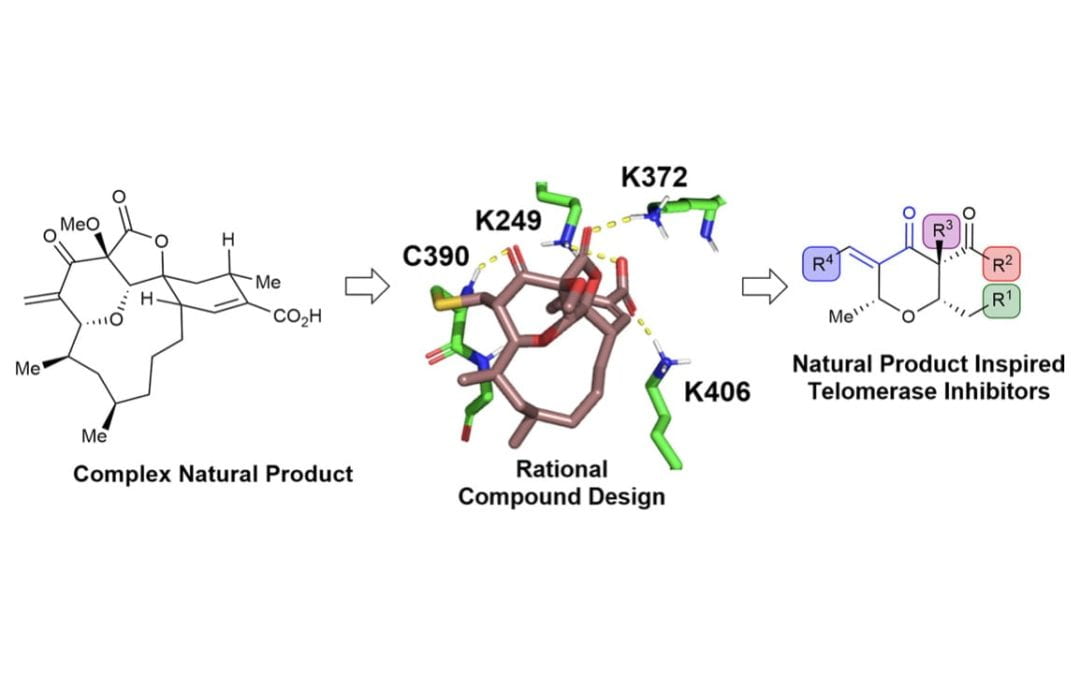
Targeted Covalent Inhibition of Telomerase
Betori, R. C.; Liu, Y.; Mishra, R. K.; Cohen, S. B.; Kron, S. J.; Scheidt, K. A.*
ACS Chem. Biol. 2020, 15, 706-717.
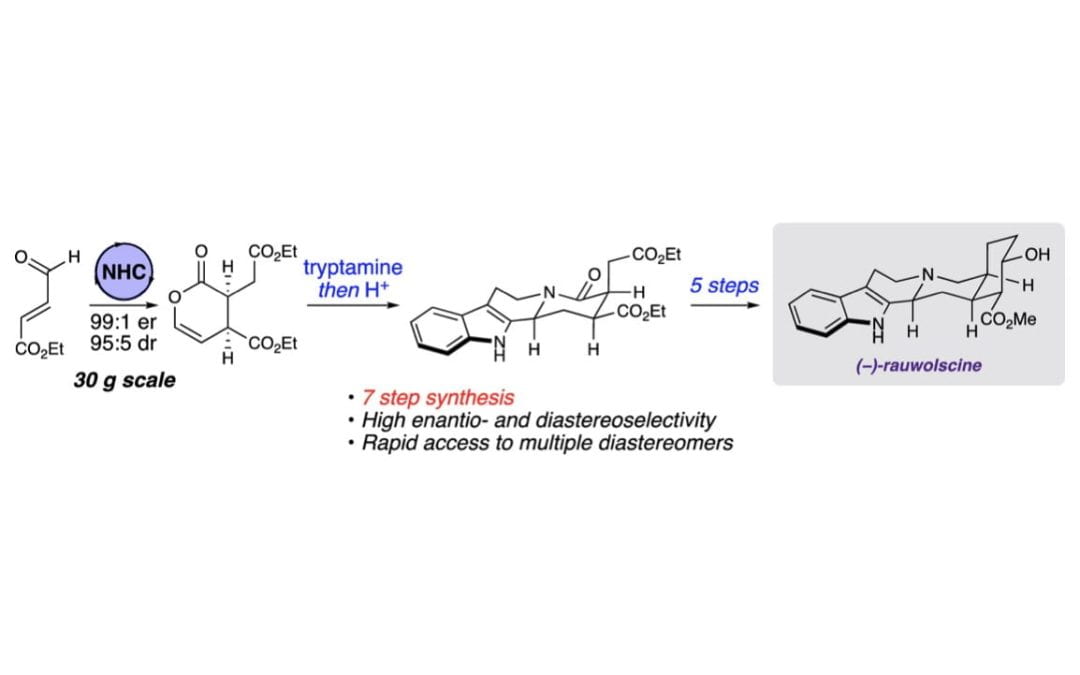
A Concise, Enantioselective Approach for the Synthesis of Yohimbine Alkaloids
Miller, E. R.; Hovey, M. T.; Scheidt, K. A.*
J. Am. Chem. Soc. 2020, 142, 2187-2192.
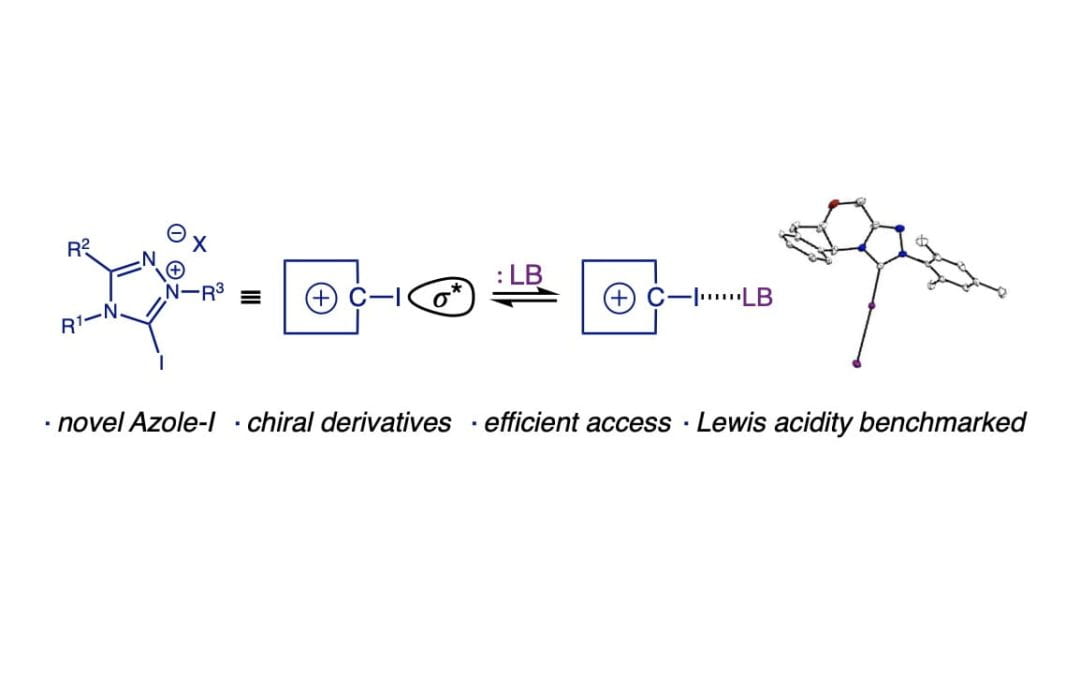
Synthesis and Evaluation of Azolium-Based Halogen Bond Donors
Squitieri, R. A.; Fitzpatrick, K. P.; Jaworski, A. A.; Scheidt, K. A.*
Chem. Eur. J. 2019, 43, 10069-10073.
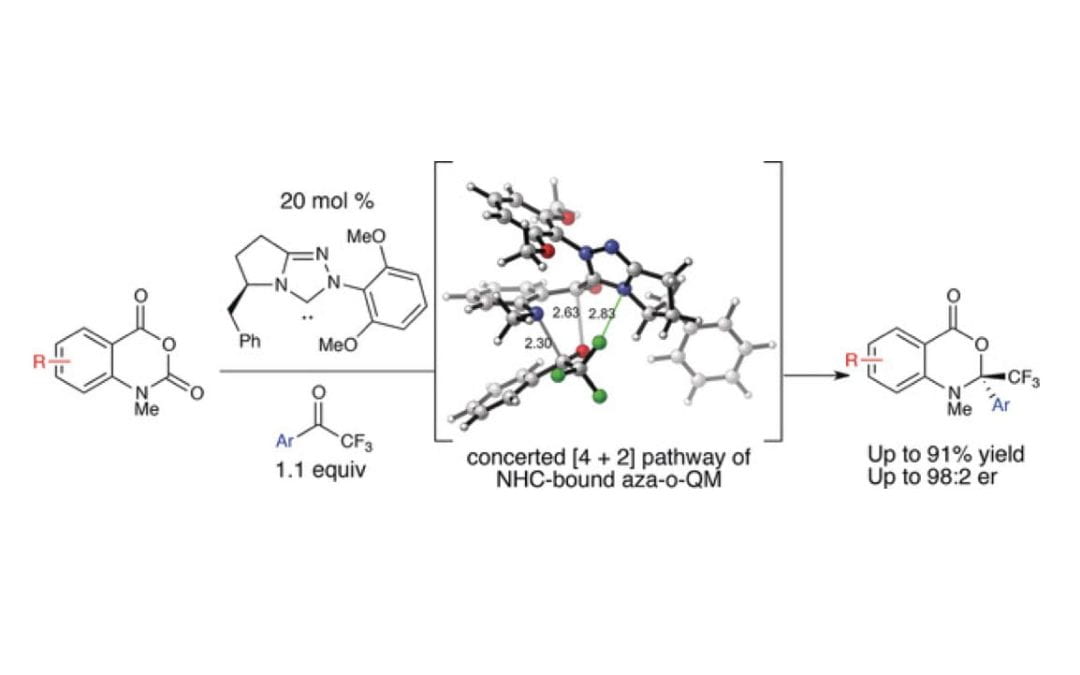
Carbene‐Catalyzed Enantioselective Decarboxylative Annulations to Access Dihydrobenzoxazinones and Quinolones
Lee, A.; Zhu, J. L.; Feoktistova, T.; Brueckner, A. C.; Cheong, P. H.-Y.; Scheidt, K. A.*
Angew. Chem. Int. Ed. 2019, 58, 5941-5945.
Featured in: List, B.; Schwengers, S. Synfacts 2019, 15 (08), 0929.
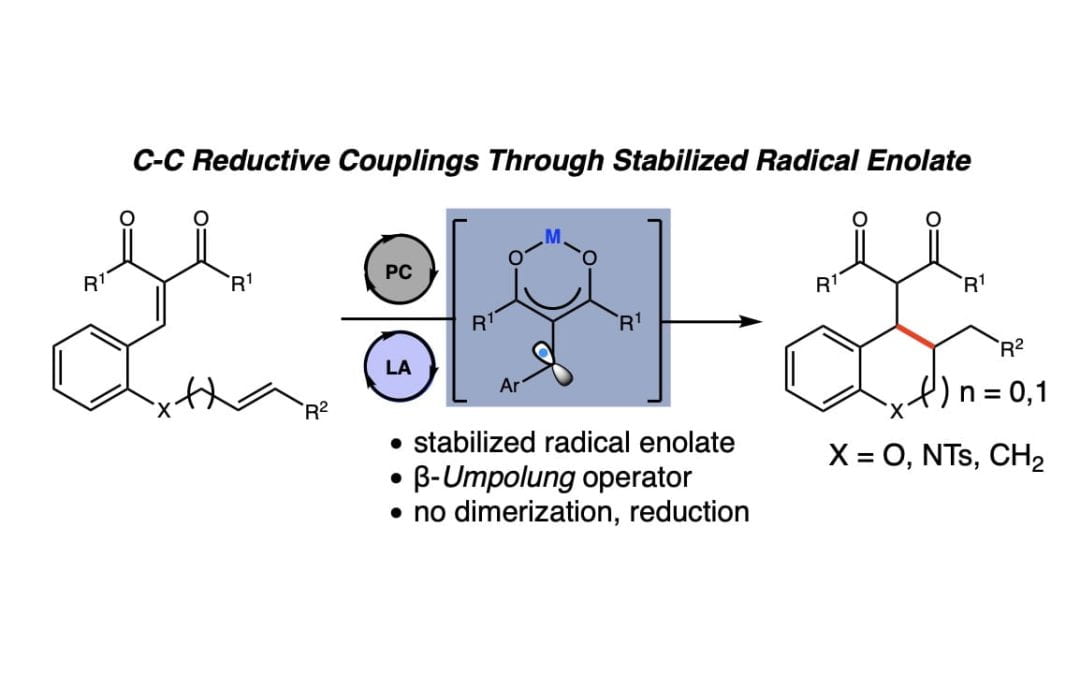
Reductive Annulations of Arylidene Malonates With Unsaturated Electrophiles Using Photoredox/Lewis Acid Cooperative Catalysis
Betori, R. C.; McDonald, B. R.; Scheidt, K. A.*
Chem. Sci. 2019, 10, 3353-3359.
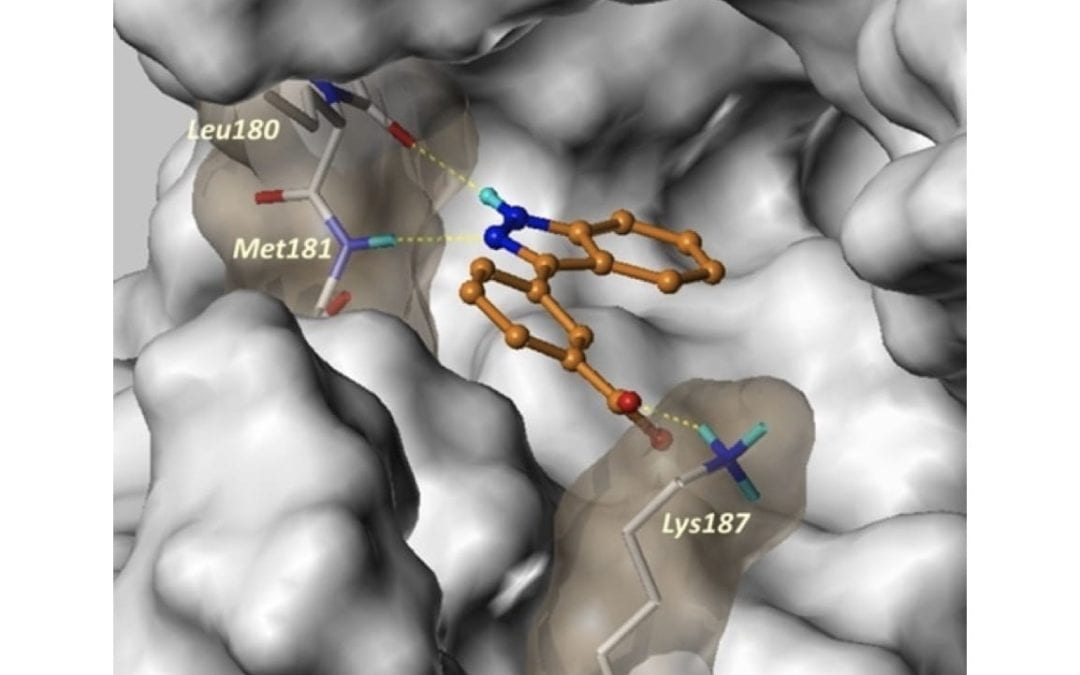
Synthesis and Biological Evaluation of 3-Arylindazoles as Selective MEK4 Inhibitors
Deibler, K. K.; Schiltz, G. E.; Clutter, M. R.; Mishra, R. K.; Vagadia, P.P.; O’Connor, M.; George, M. D.; Gordon, R.; Fowler, G.; Bergan, R.; Scheidt, K. A.*
Chem. Med. Chem. 2019, 14, 615-620.
Featured in: Special Collection on Cancer Research (Hot Topics 2020, Chem. Med. Chem.)
![NHC-Catalyzed Formal [2+2] Annulations of Allenoates for the Synthesis of Substituted Oxetanes](https://scheidtgroup.northwestern.edu/files/2021/02/abstract_156-1080x675.jpeg)
NHC-Catalyzed Formal [2+2] Annulations of Allenoates for the Synthesis of Substituted Oxetanes
Lopez, S. S.; Jaworski, A. A.; Scheidt, K. A.*
J. Org. Chem. 2018, 83, 14637-14645.
Featured in: Snieckus, V.; da Frota, L. C. R. M. Synfacts 2019, 15 (02), 0124.
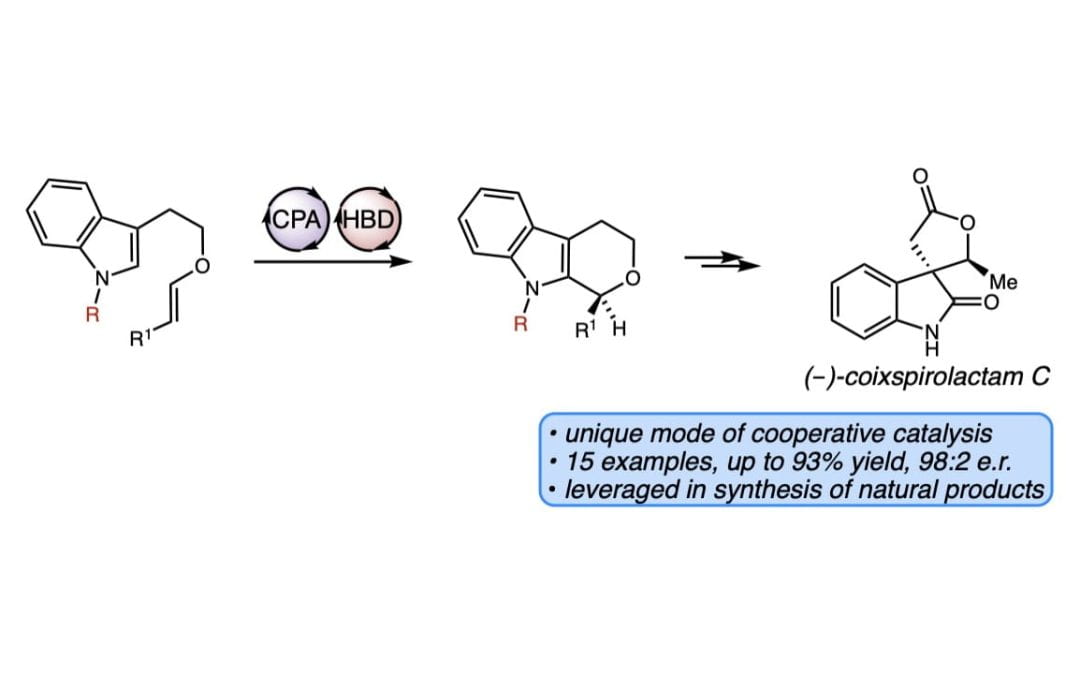
A Cooperative Hydrogen Bond Donor/Brønsted Acid System for the Enantioselective Synthesis of Tetrahydropyrans
Maskeri, M. A.; O’Connor, M. J.; Jaworski, A. A.; Bay, A. V.; Scheidt, K. A.*
Angew. Chem. Int. Ed. 2018, 57, 17225-17229.
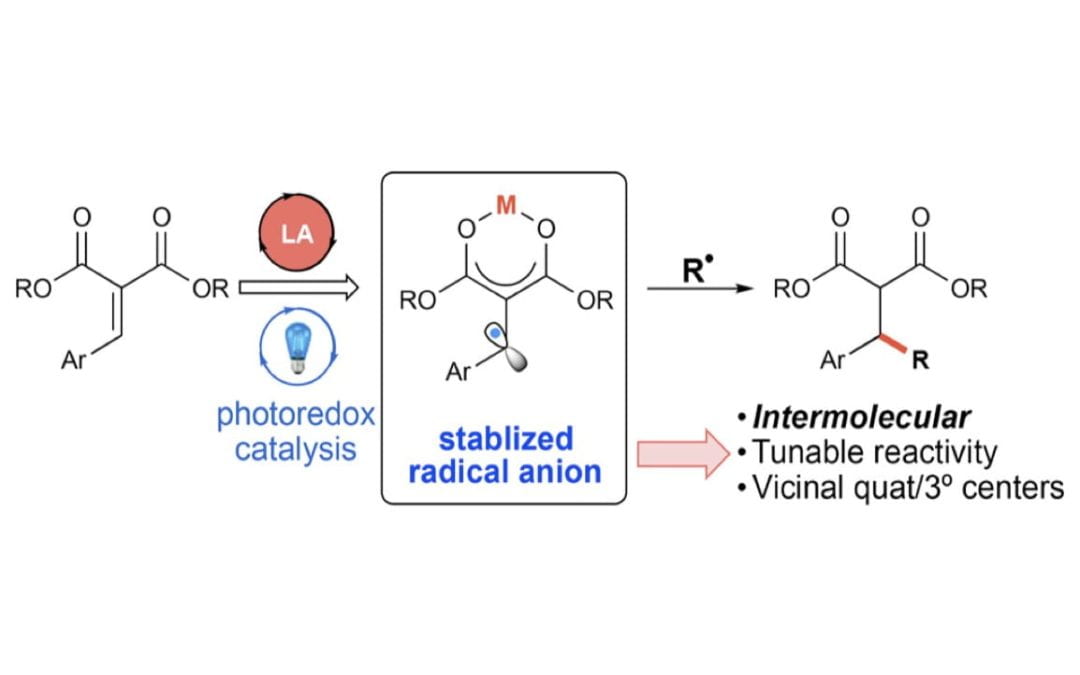
Intermolecular Reductive Couplings of Arylidene Malonates via Lewis Acid/Photoredox Cooperative Catalysis
McDonald, B. R.; Scheidt, K. A.*
Org. Lett. 2018, 20, 6877-6881.
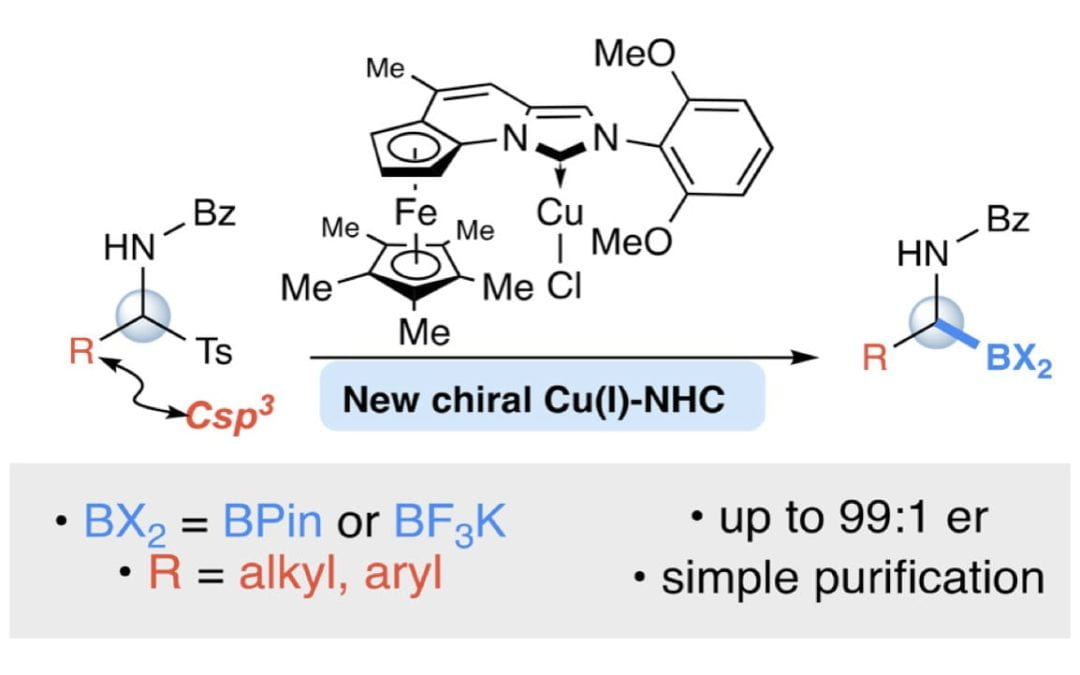
Enantioselective Synthesis of α-Amidoboronates Catalyzed by Planar-Chiral NHC-Cu(I) Complexes
Schwamb, C. B.; Fitzpatrick, K. P.; Brueckner, A. C.; Richardson, H. C.; Cheong, P. H.-Y.; Scheidt, K. A.*
J. Am. Chem. Soc. 2018, 140 (34), 10644-10648.
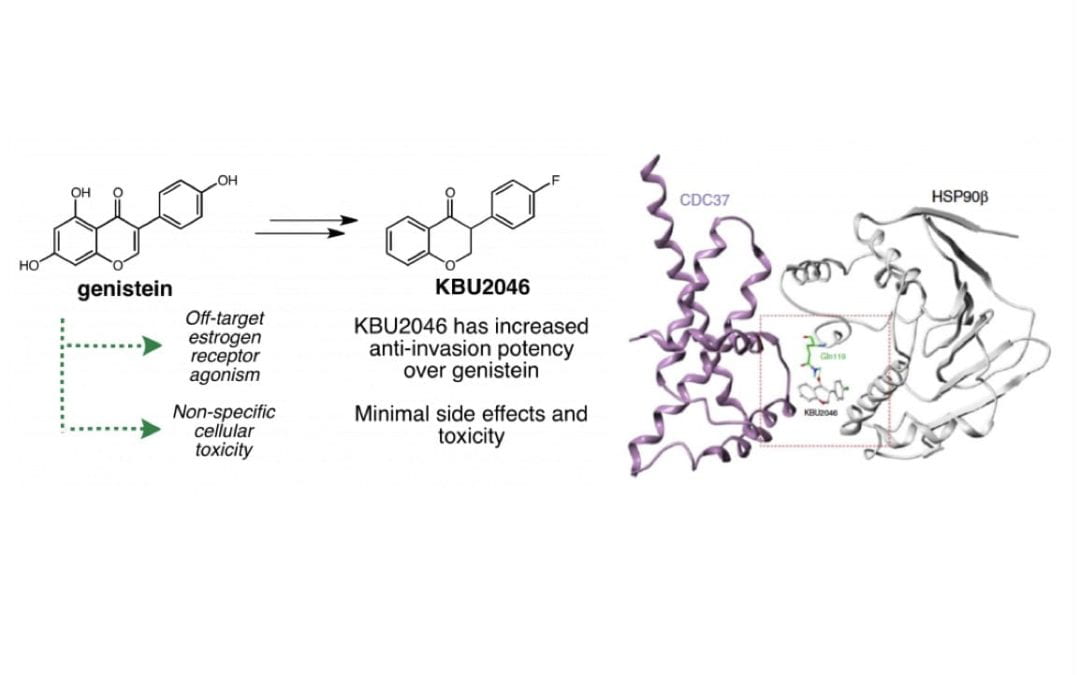
Precision therapeutic targeting of human cancer cell motility
Xu, L.; Gordon, R.; Farmer, R.; Pattanayak, A.; Binkowski, A.; Huang, X.; Avram, M.; Krishna, S.; Voll, E.; Pavese, J.; Chavez, J.; Bruce, J.; Mazar, A.; Nibbs, A.; Anderson, W.; Li, L.; Jovanovic, B.; Pruell, S.; Valsecchi, M.; Francia, G.; Betori, R. Scheidt, K.; Bergan, R.*
Nat. Commun. 2018, 9, 2454.
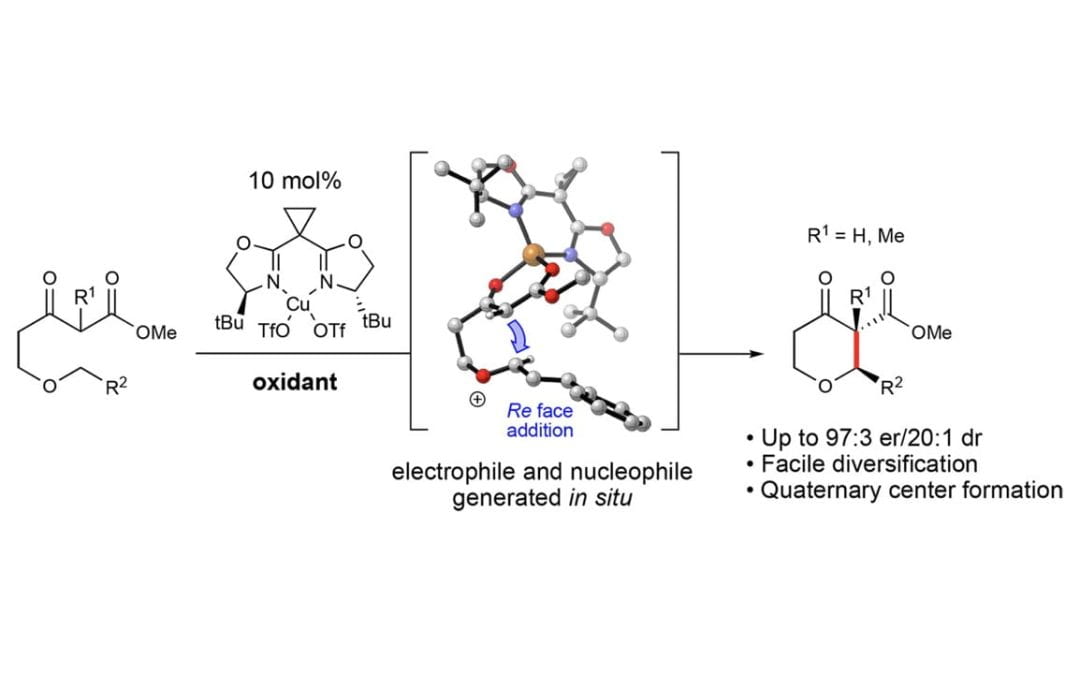
An Enantioselective Cross-Dehydrogenative Coupling Catalysis Approach to Tetrahydropyrans
Lee, A.; Betori, R. C.; Crane, E. A.; Scheidt, K. A.*
J. Am. Chem. Soc. 2018, 140 (20), 6212-6216.
Featured in: Snieckus, V.; Lombardo, V. M. Synfacts 2018, 14 (08), 0801.
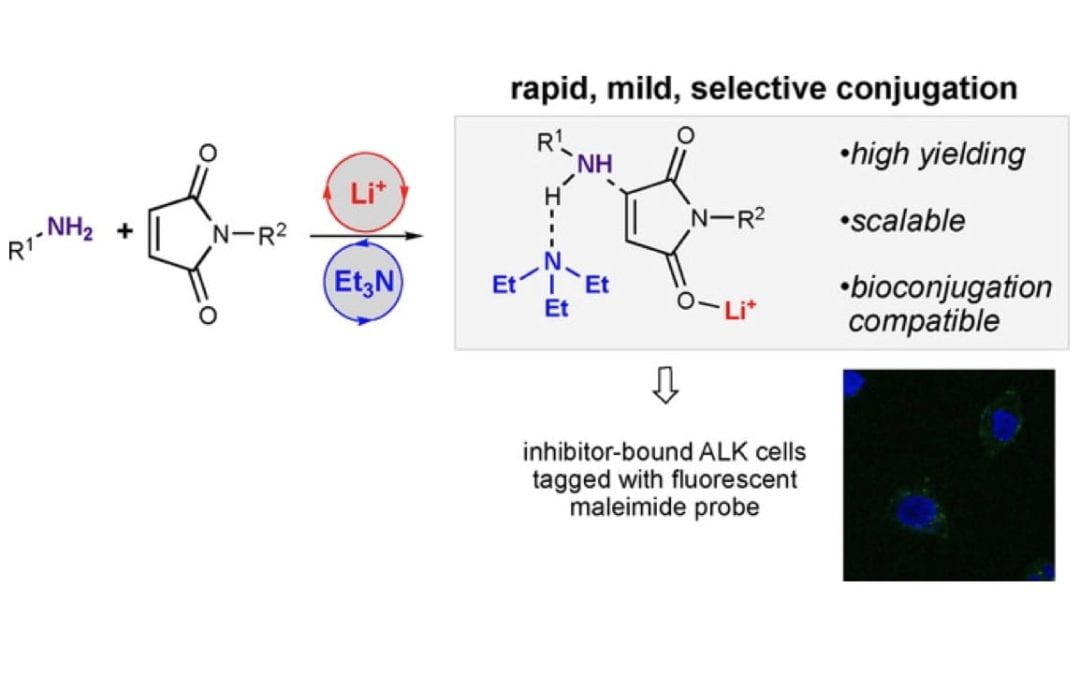
Conjugate Additions of Amines to Maleimides via Cooperative Catalysis
Uno, B. E.; Deibler, K. K.; Villa, C.; Raghuraman, A.; Scheidt, K. A.*
Adv. Synth. Catal. 2018, 360 (8), 1719-1725.
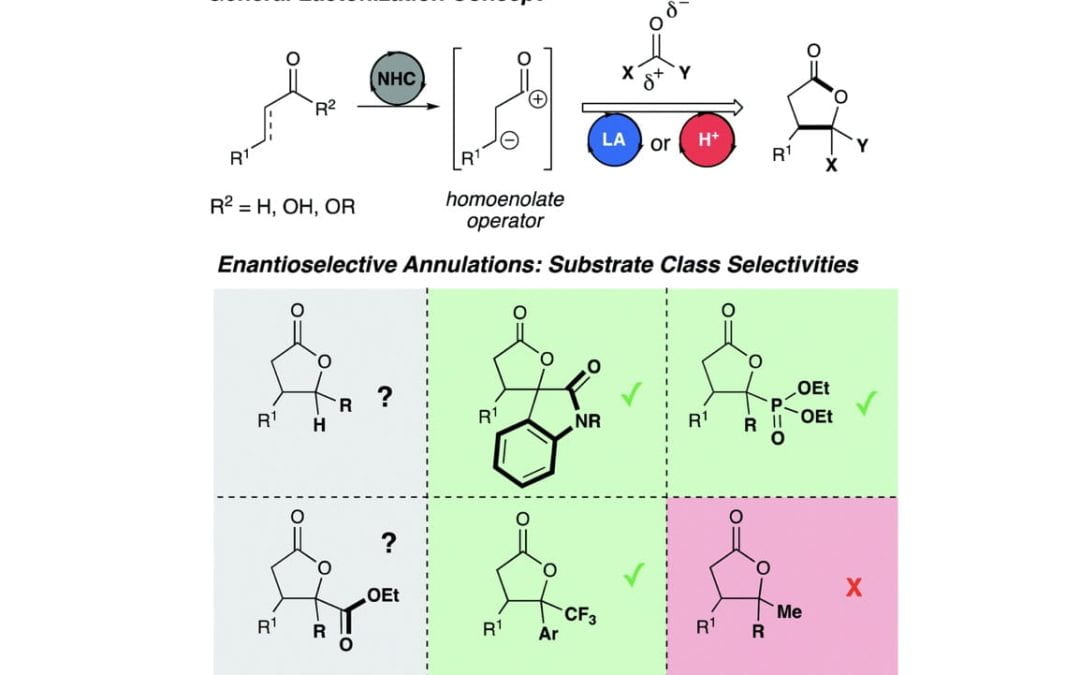
A continuing challenge: N-heterocyclic carbene-catalyzed syntheses of γ-butyrolactones
Murauski, K. J. R.; Jaworski, A. A.; Scheidt, K. A.*
Chem. Soc. Rev. 2018, 47, 1773-1782.
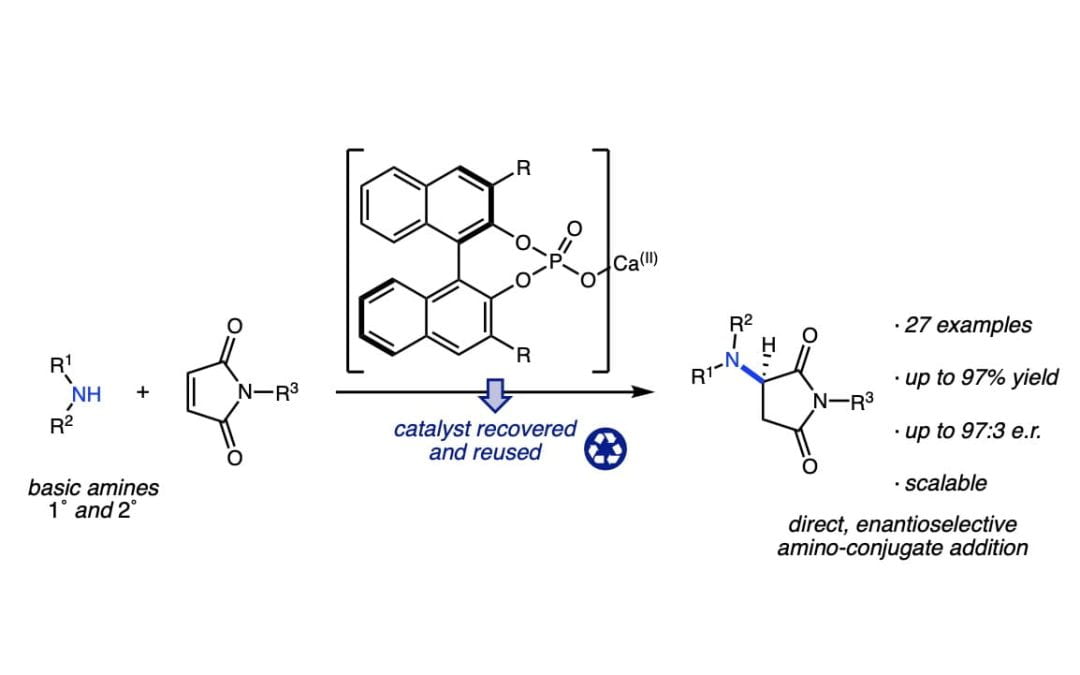
Calcium(II)-catalyzed enantioselective conjugate additions of amines
Uno, B. E.; Dicken, R. D.; Redfern, L. R.; Stern, C. M.; Krzywicki, G. G.; Scheidt, K. A.*Chem. Sci. 2018, 9, 1634-1639.
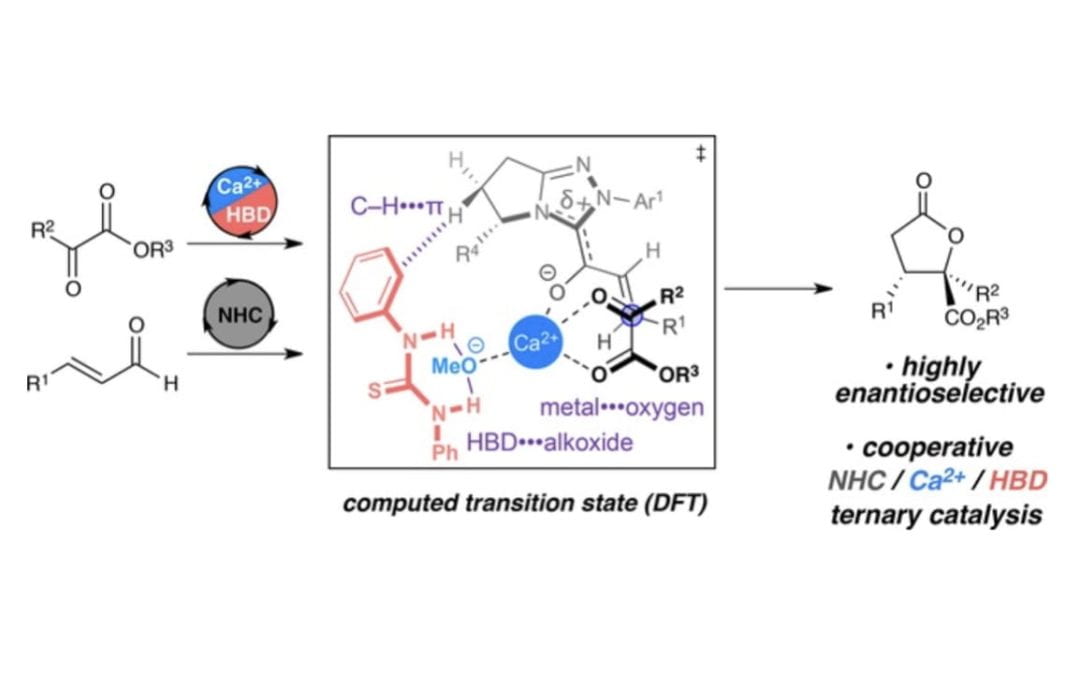
A Cooperative Ternary Catalysis System for Asymmetric Lactonizations of α-Ketoesters
Murauski, K. J. R.; Walden, D. M.; Cheong, P. H-Y.; Scheidt, K. A.*
Adv. Synth. Catal. 2017, 359, 3713-3719. https://doi.org/10.1002/adsc.201701015
Featured in: Yamamoto, H.; Shimoda, Y. Synfacts 2018, 14 (02), 0163.
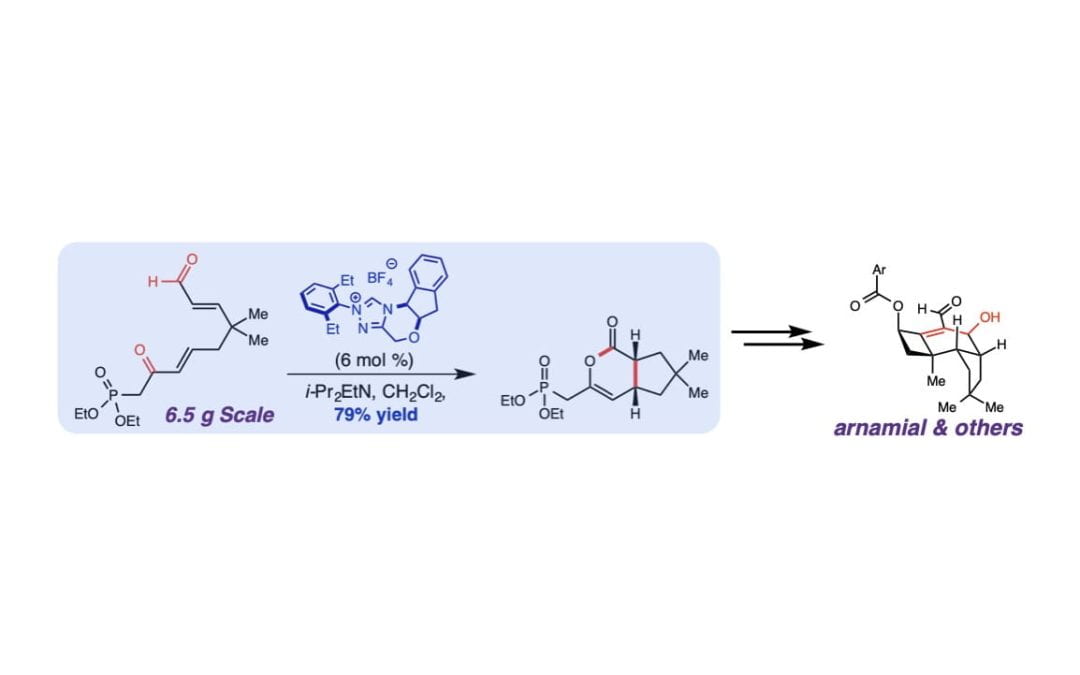
A Carbene Catalysis Strategy for the Synthesis of Protoilludane Natural Products
Hovey, M. T.; Cohen, D. T.; Walden, D. M.; Cheong, P. H-Y.; Scheidt , K. A.*
Angew. Chem. Int. Ed. 2017 , 56, 9864-9867.
Featured in: Carreira, E. M.; Sievertsen, N. Synfacts 2017, 13 (10), 1012.
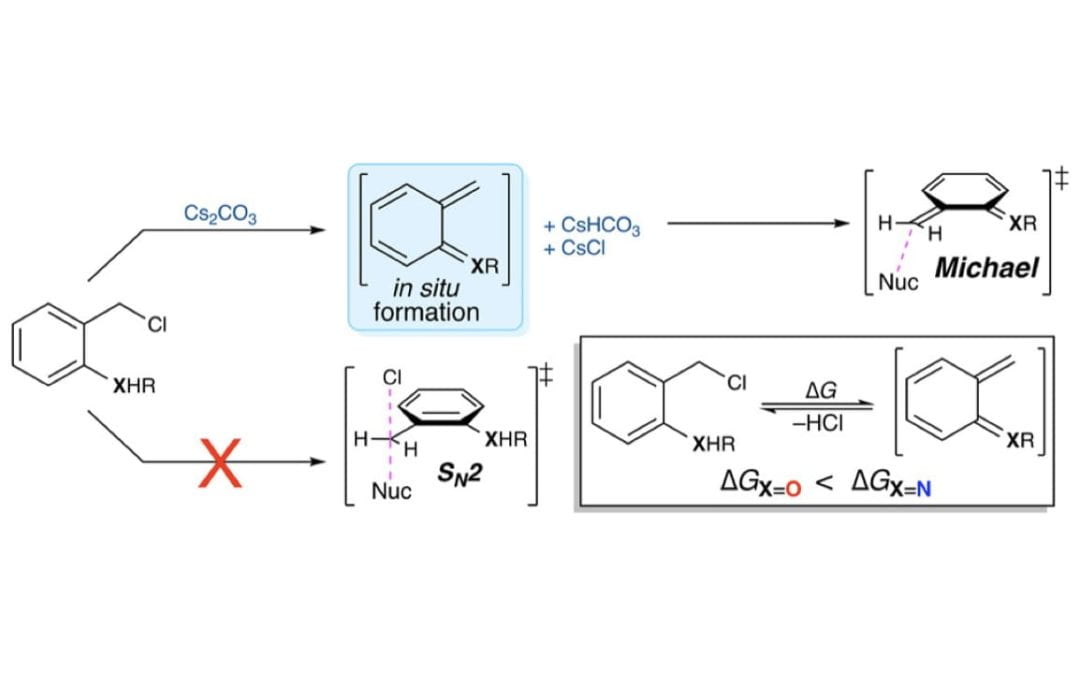
Formation of Aza-ortho-quinone Methides Under Room Temperature Conditions: Cs2CO3 Effect
Walden, D. M.; Johnston, R. C.; Jaworski, A. A.; Hovey, M. T.; Meyer, M. P.; Scheidt, K. A.* and Cheong, P. H.*
J. Org. Chem. 2017, 82, 7183-7189
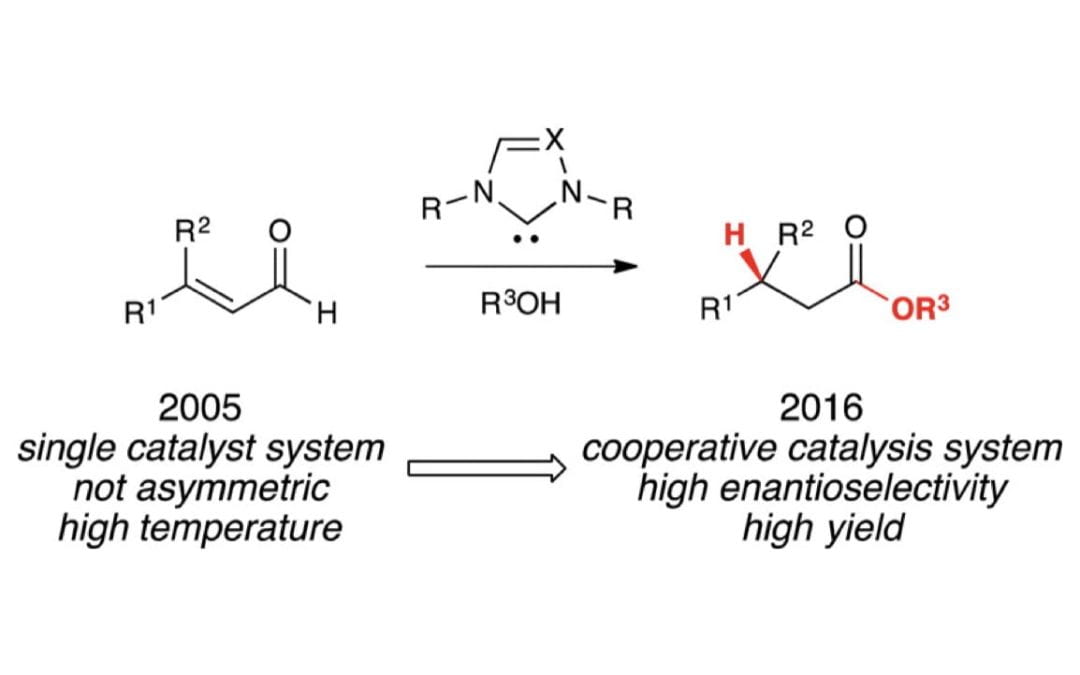
Catalytic, Enantioselective β-Protonation through a Cooperative Activation Strategy
Wang , M.; Barsoum , D.; Schwamb , C. B.; Cohen, D. T.; Goess , B. C.; Riedrich , M.; Chan, A.; Maki, B. E.; Mishra, R. K.; Scheidt , K. A.*
J. Org. Chem. 2017, 82, 4689-4702. https://doi.org/10.1021/acs.joc.7b00334
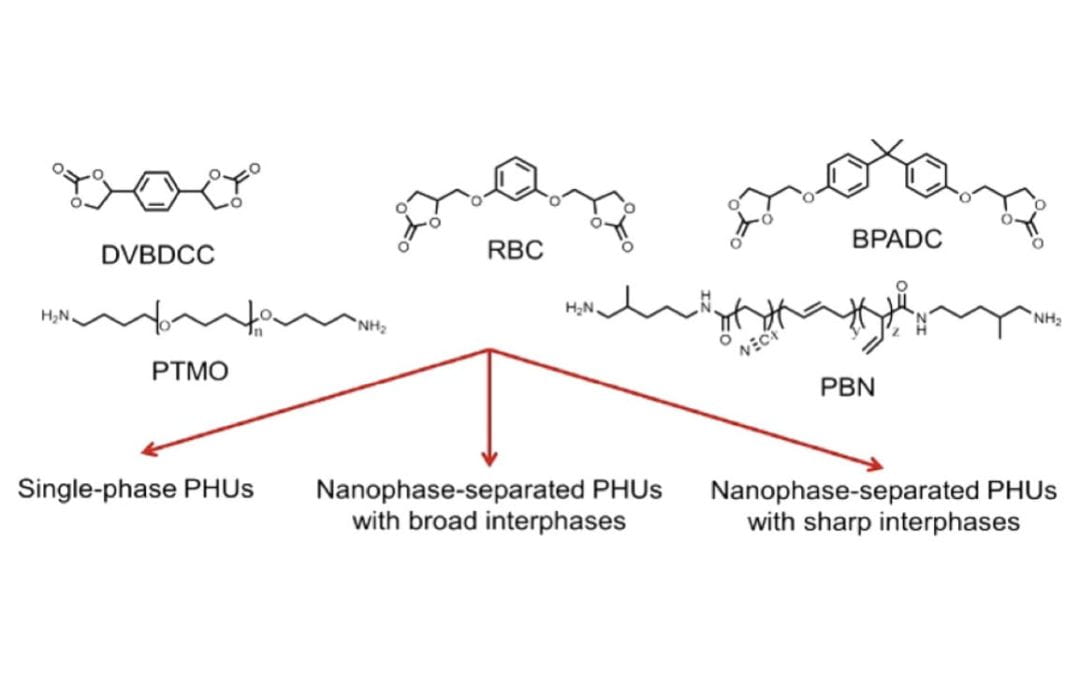
Combined Effects of Carbonate and Soft-Segment Molecular Structures on the Nanophase Separation and Properties of Segmented Polyhydroxyurethane
Beniah, G.; Chen, X.; Uno, B. E.; Liu, K.; Leitsch, E. K.; Jeon, J.; Heath, W. H.; Scheidt, K. A.; Torkelson, J. M.*
Macromolecules 2017, 50, 3193-3203. https://doi.org/10.1021/acs.macromol.6b02513

A Chemical Probe Strategy for Interrogating Inhibitor Selectivity Across the MEK Kinase Family
Deibler, K. K.; Mishra, R.K.; Clutter, M. R.; Antanasijevic, A. Bergan, R.; Caffrey, M.; Scheidt, K. A.*
ACS Chem. Biol. 2017, 12, 1245-1256.

A Biocatalytic Route to Highly Enantioenriched β-Hydroxydioxinones
Betori, R. C.; Miller, E. R.; Scheidt, K. A.,
Adv. Synth. Cat. 2017, 359, 1131-1137.
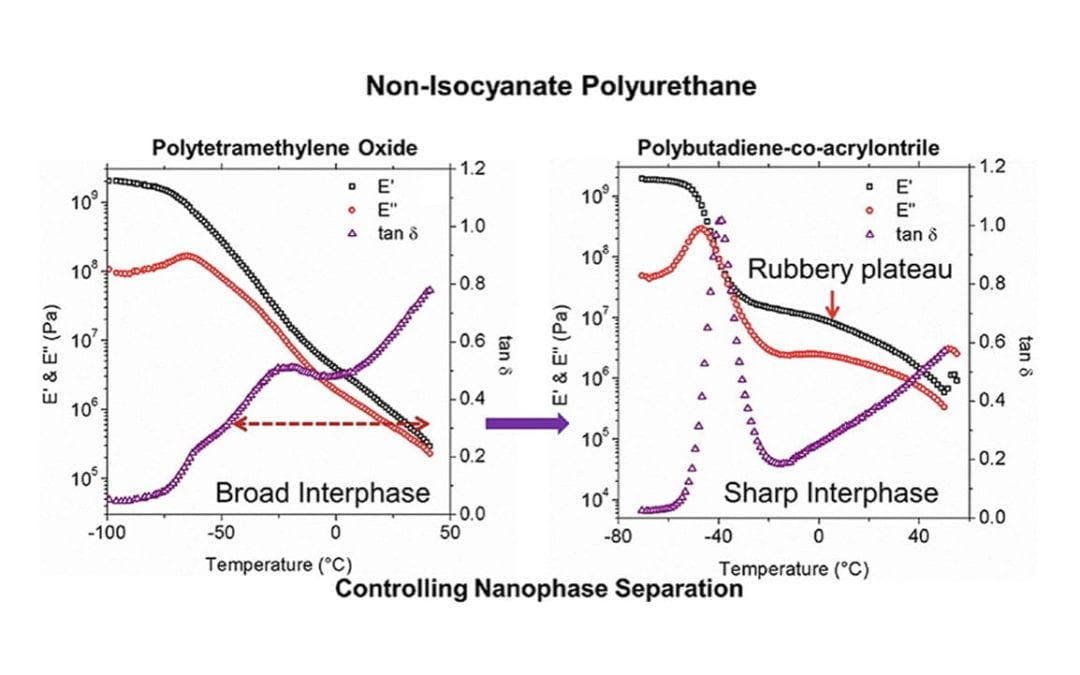
Tuning Nanophase Separation Behavior in Segmented Polyhydroxyurethane via Judicious Choice of Soft Segment
Beniah, G.; Uno, B. E.; Lan, T.; Jeon, J.; Heath, W. H.; Scheidt, K. A.; Torkelson, J. M.
Polymer 2017, 110 , 218-227 .
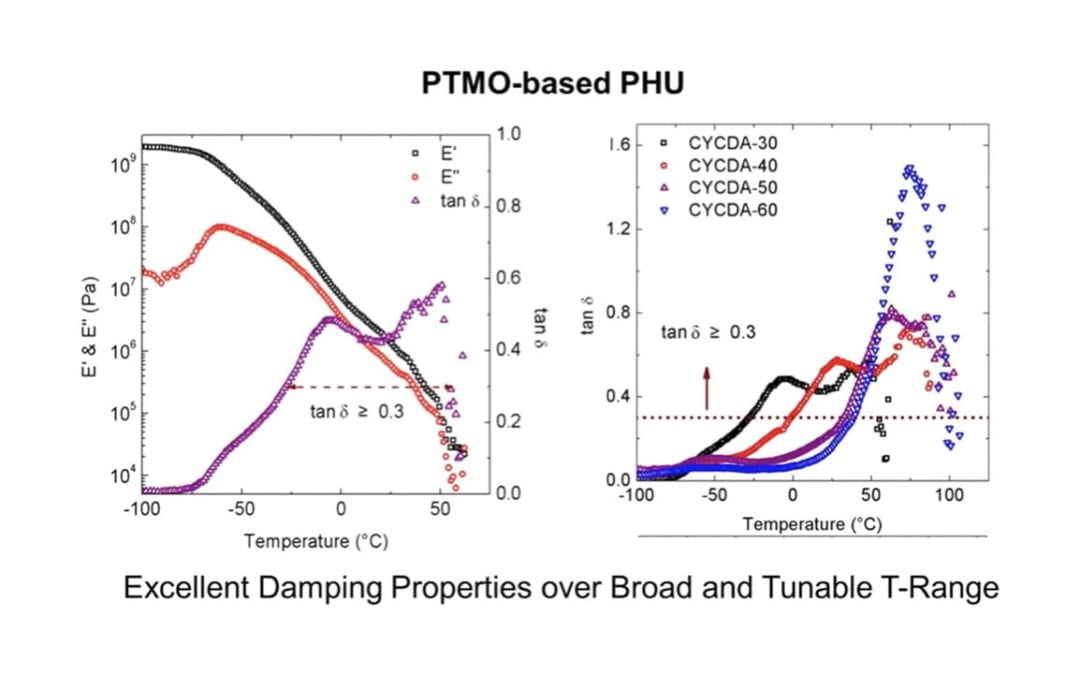
Novel Thermoplastic Polyhydroxyurethane Elastomers as Effective Damping Materials over Broad Temperature Ranges
Beniah, G. Liu, K.; Heath, W. H.; Miller, M. D.; Scheidt, K. A.; Torkelson, J. M.*
Eur. Polymer J. 2016 , 84, 770-783.
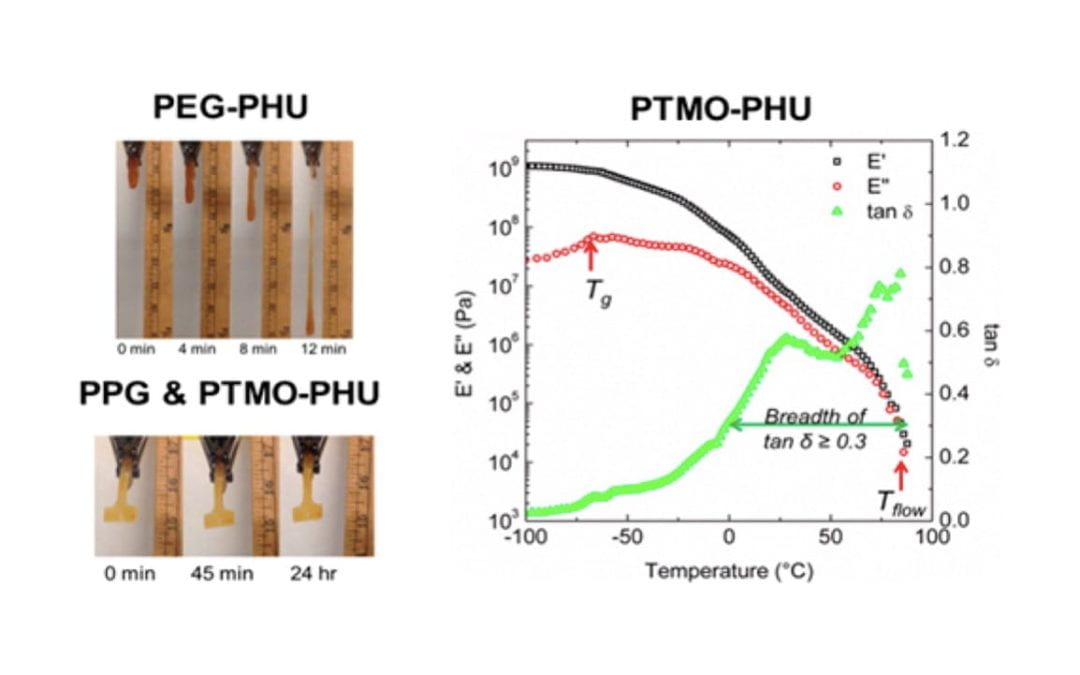
Non-Isocyanate Thermoplastic Polyhydroxyurethane Elastomers via Cyclic Carbonate Aminolysis: Critical Role of Hydroxyl Groups in Controlling Nanophase Separation
Leitsch, E.; Beniah, G. Liu, K.; Lan, T.; Heath, W.; Scheidt, K. A.; Torkelson, J.*
ACS Macro Letters 2016, 5, 424-429.
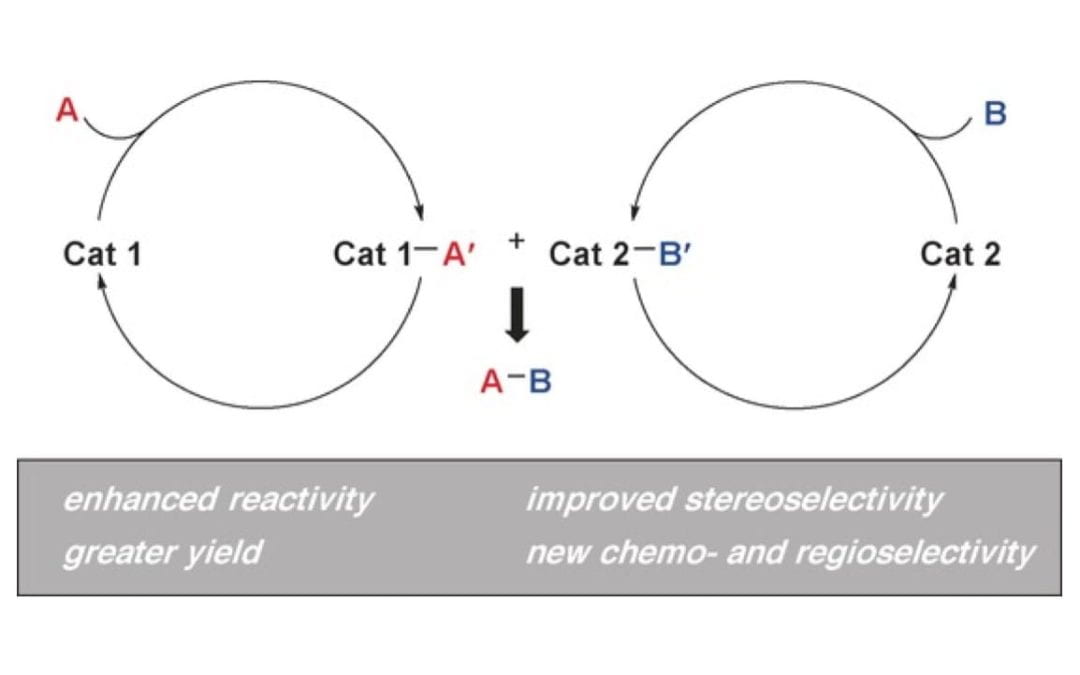
Cooperative Catalysis and Activation with N-Heterocyclic Carbenes
Wang, M. H.; Scheidt, K. A.,
Angew. Chem. Int. Ed. 2016, 5, 424-429.
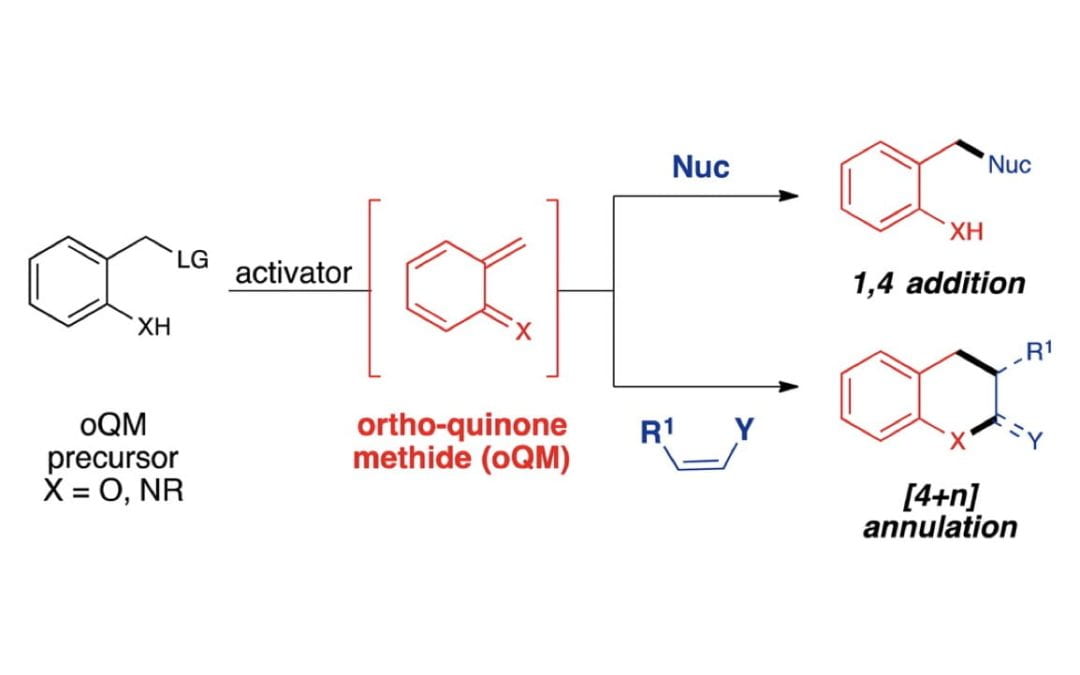
Emerging Roles of in Situ Generated Quinone Methides in Metal-Free Catalysis
Jaworski, A. A.; Scheidt, K. A.
J. Org. Chem. 2016, 81, 10145-10153.
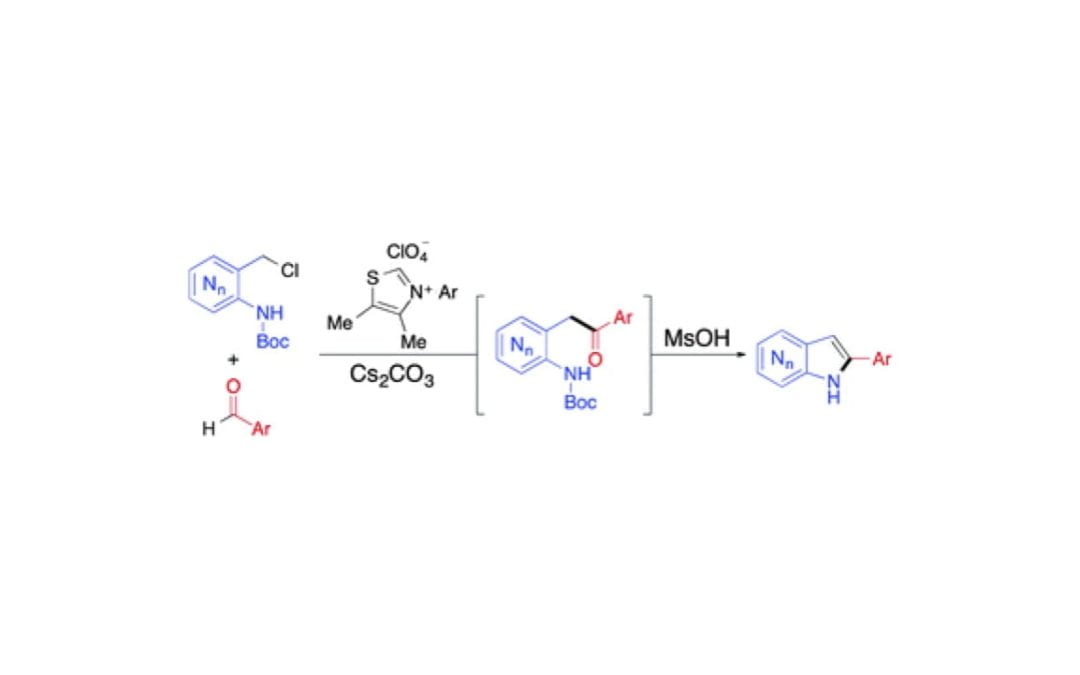
Azaindole synthesis through dual activation catalysis with N-heterocyclic carbenes
Sharma, H. A.; Hovey, M. T.; Scheidt, K. A.
Chem. Commun. 2016, 52, 9283-9286.
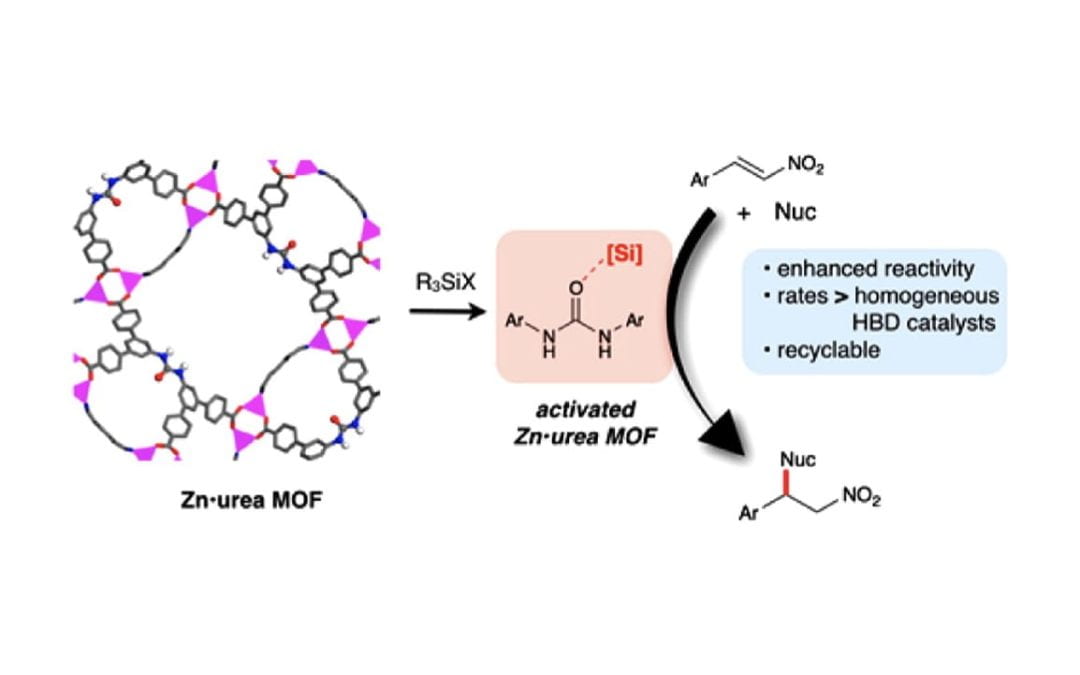
Lewis Acid Activation of a Hydrogen Bond Donor Metal–Organic Framework for Catalysis
Hall, E. A.; Redfern, L. R.; Wang, M. H.; Scheidt, K. A.
ACS Catal. 2016, 6, 3248-3252.
Featured in: Uozumi, Y.; Yamada, Y. M. A.; Ishii, R. Synfacts 2016, 12 (8), 875.
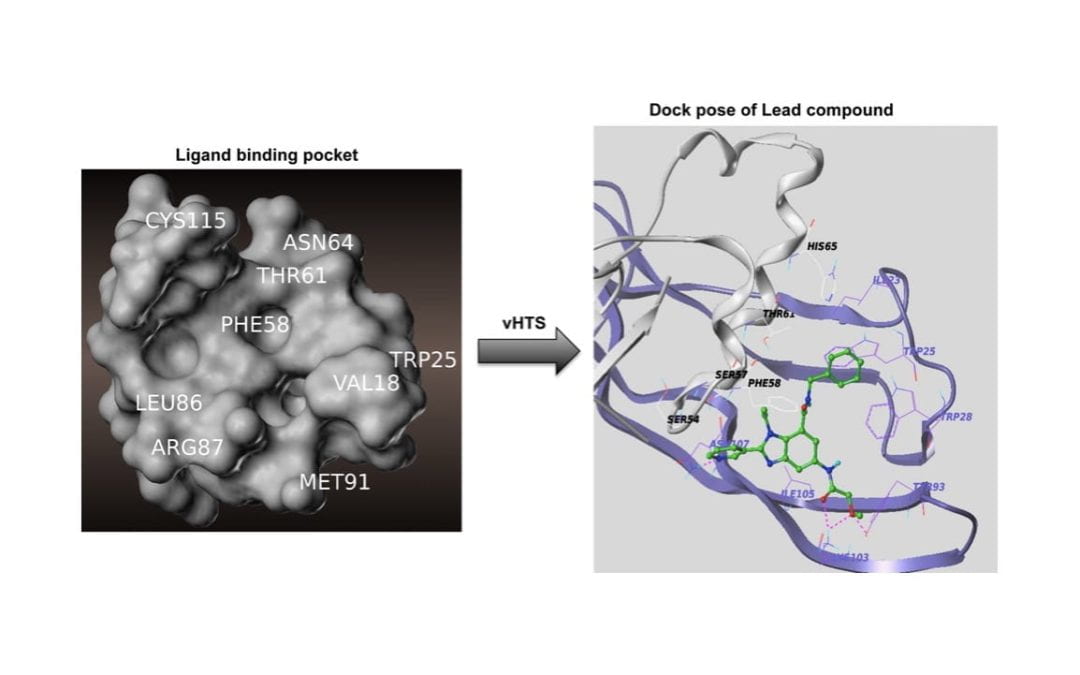
Virtual High-Throughput Screening To Identify Novel Activin Antagonists
Zhu, J.; Mishra, R. K.; Schiltz, G. E.; Makanji, Y.; Scheidt, K. A.; Mazar, A. P.; Woodruff, T. K.
J. Med. Chem. 2015, 58 (14), 5637-5648.
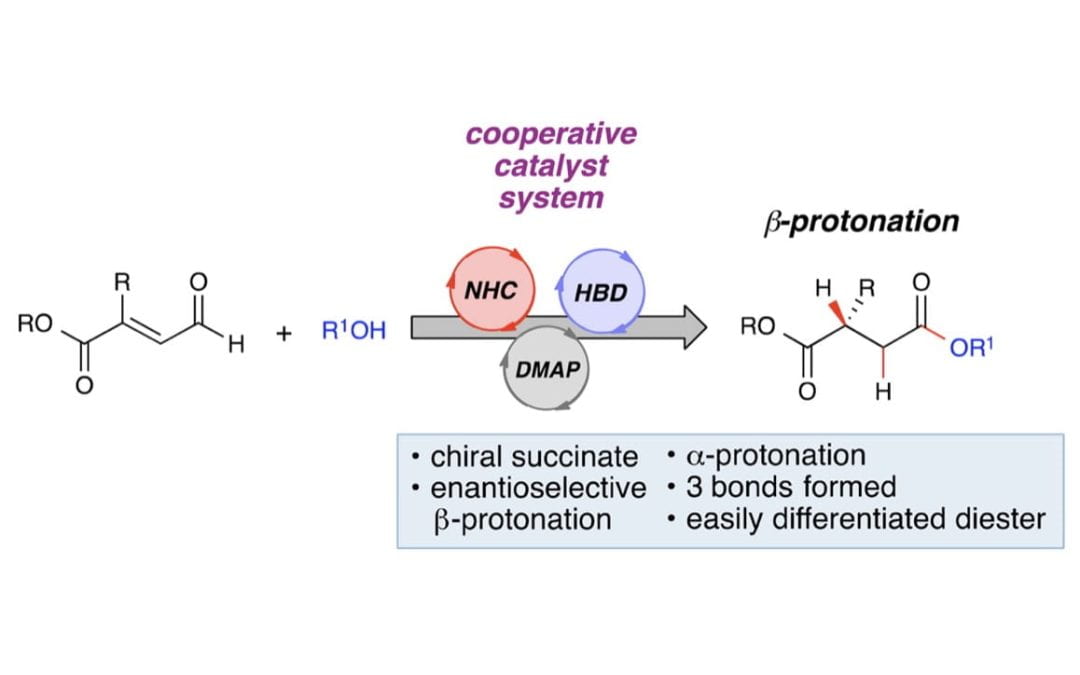
Enantioselective β-Protonation by a Cooperative Catalysis Strategy
Wang, M. H.; Cohen, D.T.; Schwamb, C. B. Mishra, R. K.; Scheidt, K. A.
J. Am. Chem. Soc. 2015, 137, 5891-5894.
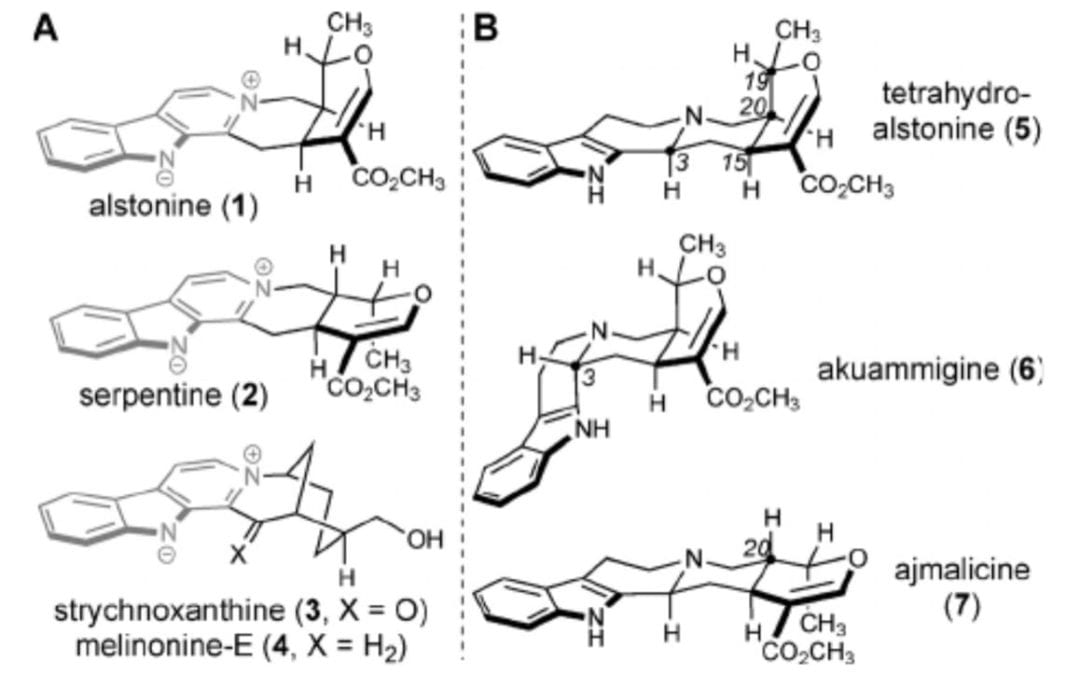
Enantioselective Syntheses of Heteroyohimbine Natural Products: A Unified Approach through Cooperative Catalysis
Younai, A.; Zeng, B.-S.; Meltzer, H. Y.; Scheidt, K. A.
Angew. Chem. Int. Ed. 2015, 54, 6900-6904.
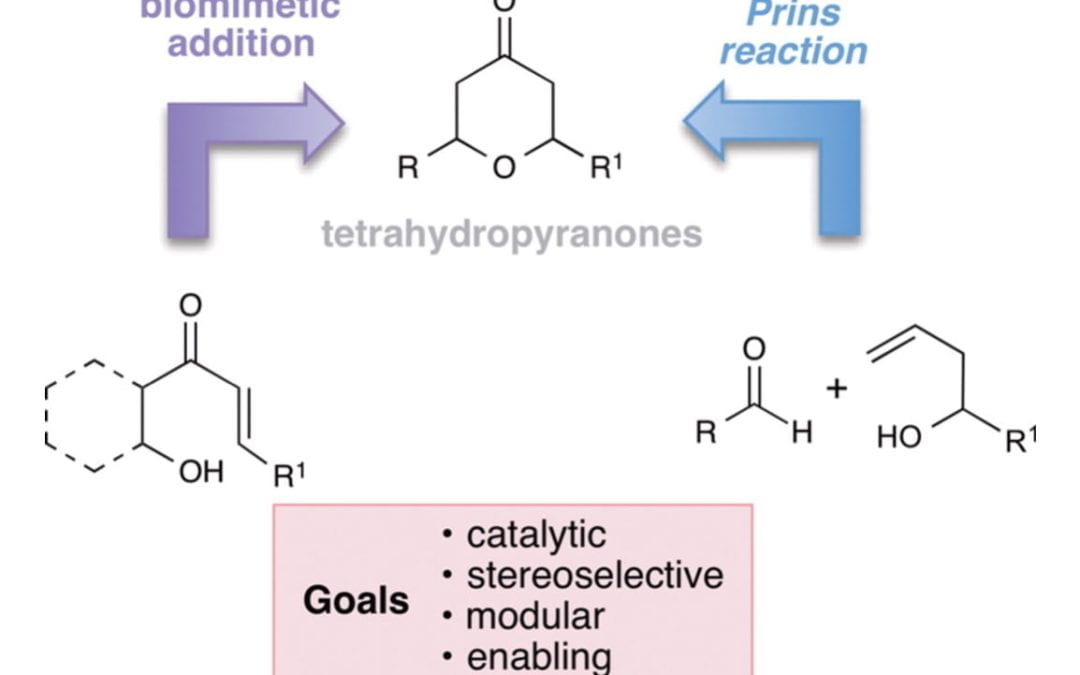
Pyranone Natural Products as Inspirations for Catalytic Reaction Discovery and Development
McDonald, B. R.; Scheidt, K. A.
Acc. Chem. Res. 2015, 48 (4), 1172-1183.
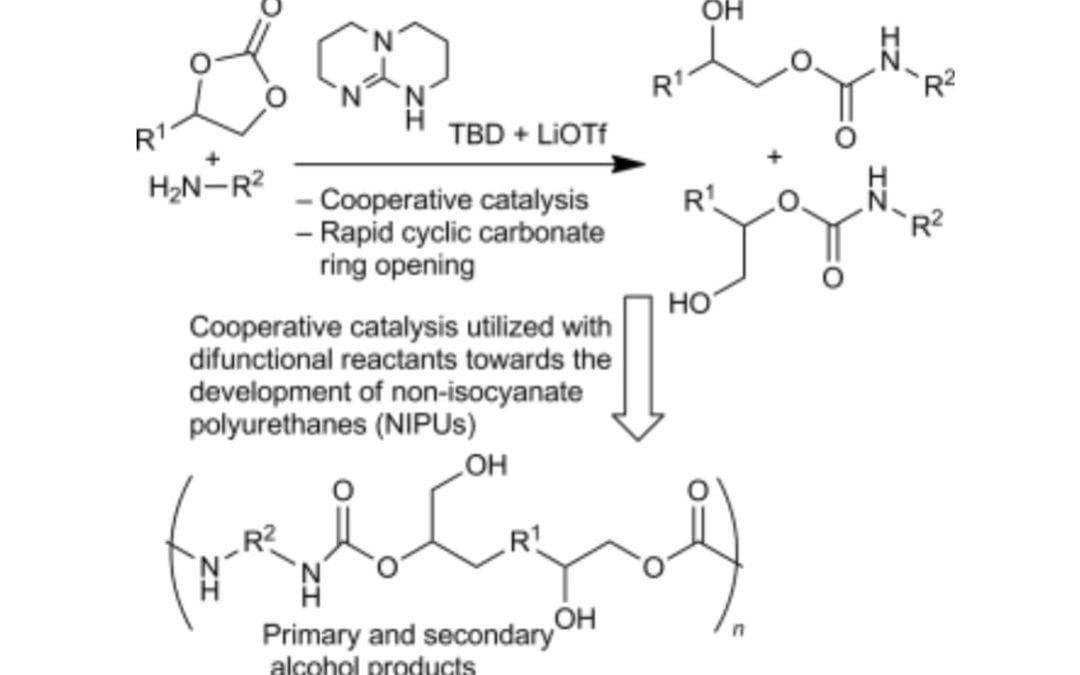
Cooperative Catalysis of Cyclic Carbonate Ring Opening: Application Towards Non-Isocyanate Polyurethane Materials
Lombardo, V. M., Dhulst, E. A.; Leitch, E. K.; Wilmot, N.; Heath, W. H.; Gies, A. P.; Miller, M. D.; Torkelson, J. M.; Scheidt, K. A.
Eur. J. Org. Chem. 2015, 2791-2795.
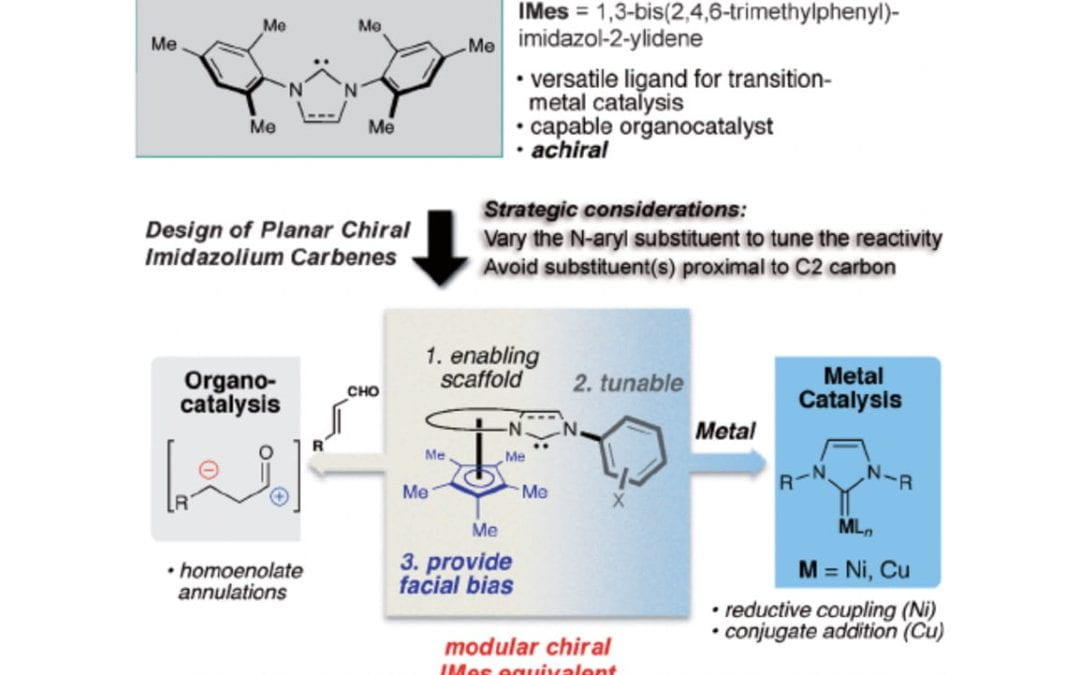
Ferrocene-Based Planar Chiral Imidazopyridinium Salts for Catalysis
Check, C. T.; Jang, K. P.; Schwamb, C. B.; Wong, A. S.; Wang, M. H.; Scheidt, K. A.
Angew. Chem. Int. Ed. 2015, 54, 4264-4268.
![N-Heterocyclic Carbene-Catalyzed Enantioselective Annulations: A Dual Activation Strategy for a Formal [4+2] Addition for Dihydrocoumarins](https://scheidtgroup.northwestern.edu/files/2021/02/abstract_127-1080x675.jpeg)
N-Heterocyclic Carbene-Catalyzed Enantioselective Annulations: A Dual Activation Strategy for a Formal [4+2] Addition for Dihydrocoumarins
Lee, A.; Scheidt, K. A.
Chem. Commun. 2015, 51, 3407-3410.
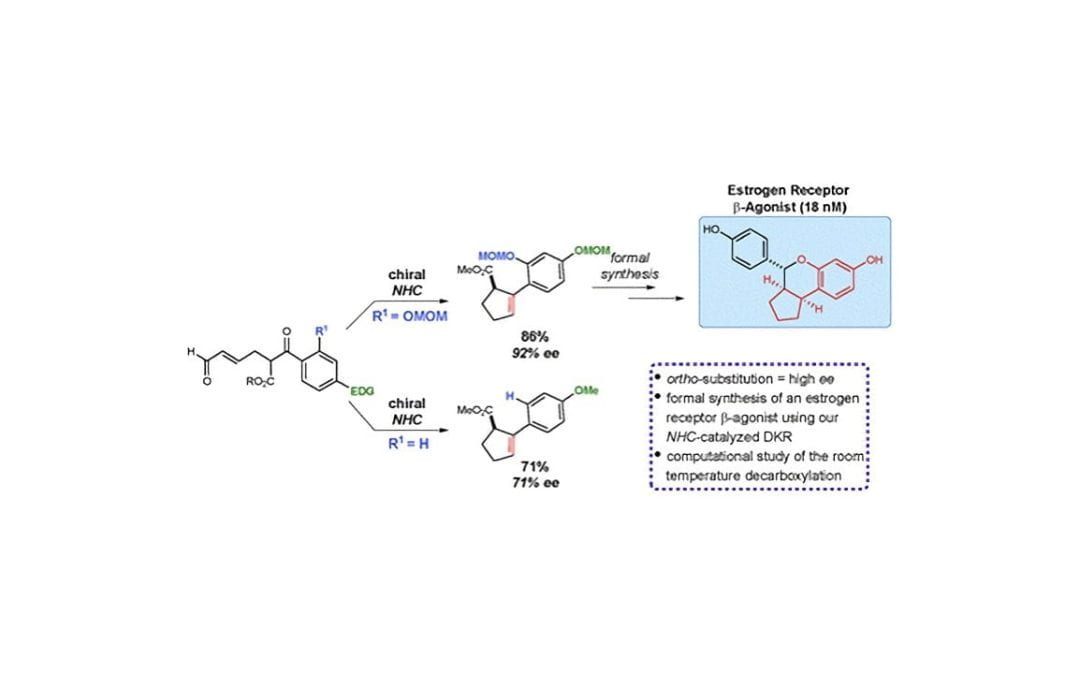
Functionalized cyclopentenes through a tandem NHC-catalyzed dynamic kinetic resolution and ambient temperature decarboxylation: mechanistic insight and synthetic application
Cohen, D. T.; Johnston, R. C.; Rosson, N. T.; Cheong, P. H.-Y.; Scheidt, K. A.
Chem. Commun. 2015, 51, 2690-2693.
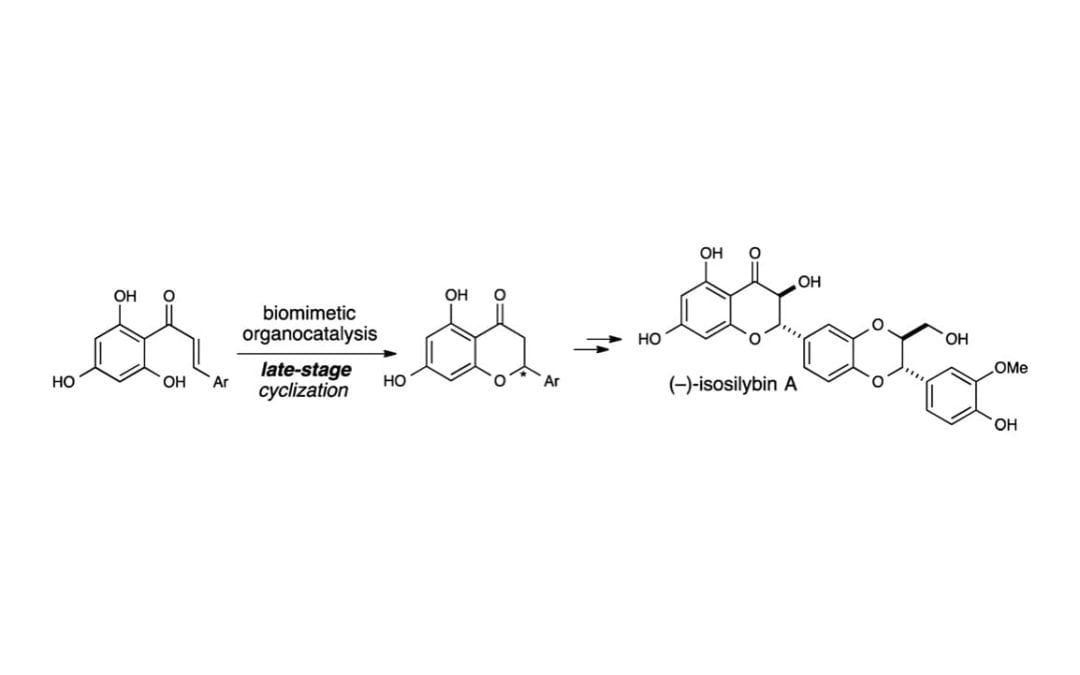
A Biomimetic Strategy to Access the Silybins: Total Synthesis of (—)-Isosilybin A
McDonald, B. R.; Nibbs, A. E.; Scheidt, K. A.
Org. Lett. 2015, 17 (1), 98-101.
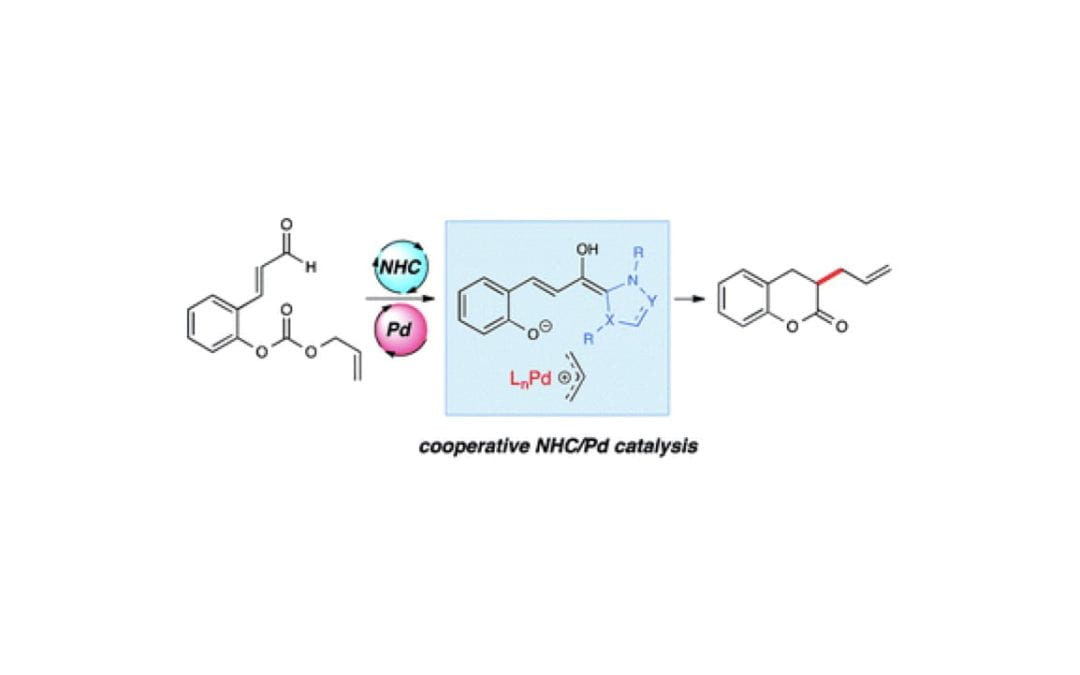
A Cooperative N-Heterocyclic Carbene/Palladium Catalysis System
Liu, K.; Hovey, M. T.; Scheidt, K. A.
Chem. Sci. 2014, 5 (10), 4026-4031.
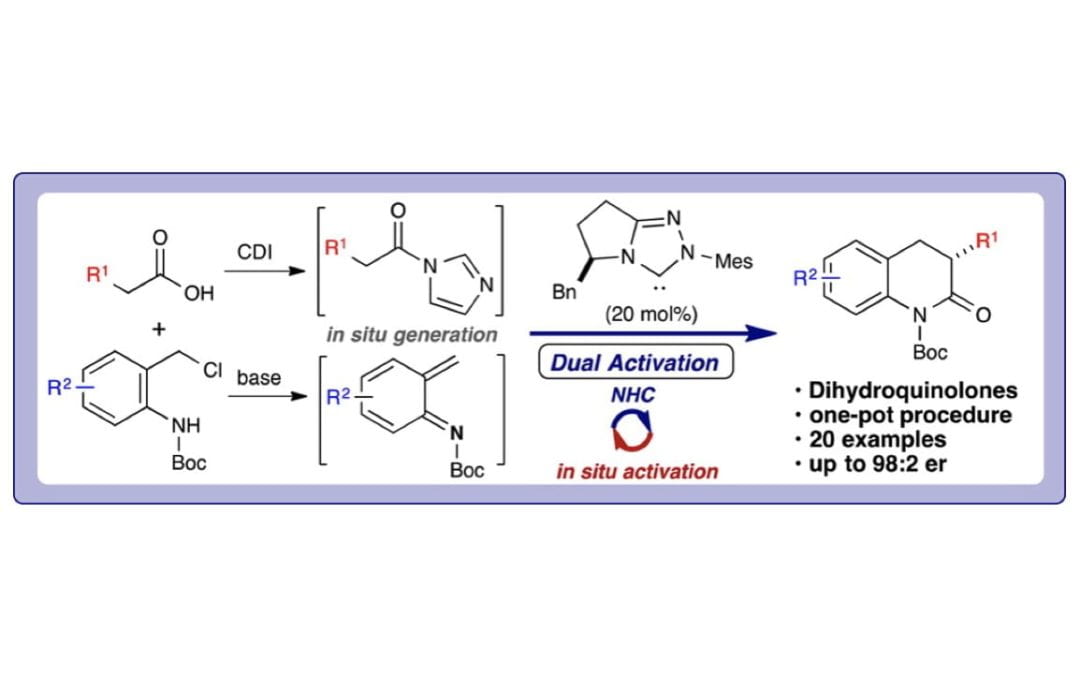
Enantioselective Annulations for Dihydroquinolones by in Situ Generation of Azolium Enolates
Lee, A.; Younai, Y.; Price, C. K.; Izquierdo, J.; Mishra, R. K.; Scheidt, K. A.
J. Am. Chem. Soc. 2014, 136 (30), 10589-10592.
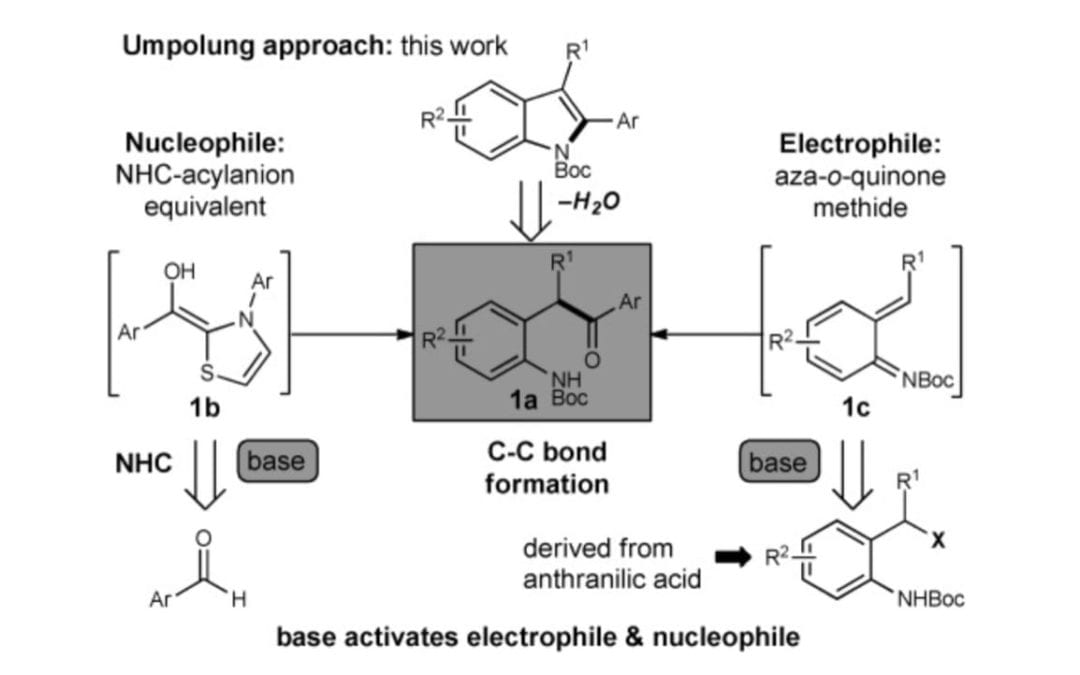
N-Heterocyclic-Carbene-Catalyzed Synthesis of 2-Aryl Indoles
Hovey, M. T.; Check, C. T.; Sipher, A. F.; Scheidt, K. A.
Angew. Chem. Int. Ed. 2014, 53, 9603–9607.
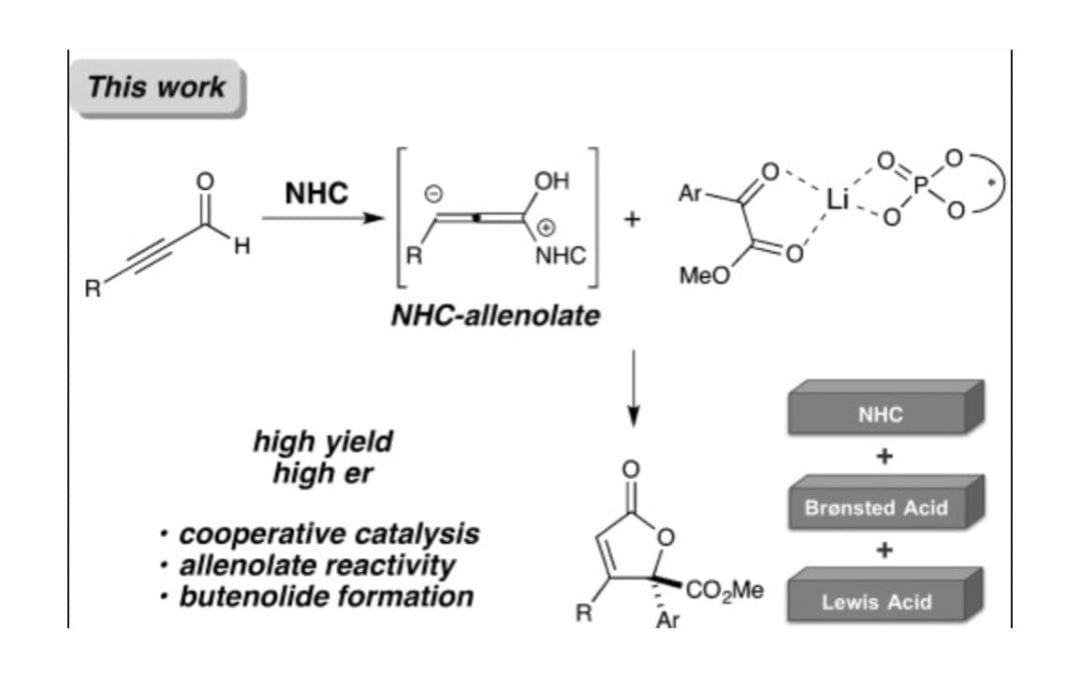
A Cooperative N-Heterocyclic Carbene/Chiral Phosphate Catalysis System for Allenolate Annulations
Lee, A.; Scheidt, K. A.
Angew. Chem. Int. Ed. 2014, 53, 7594-7598.
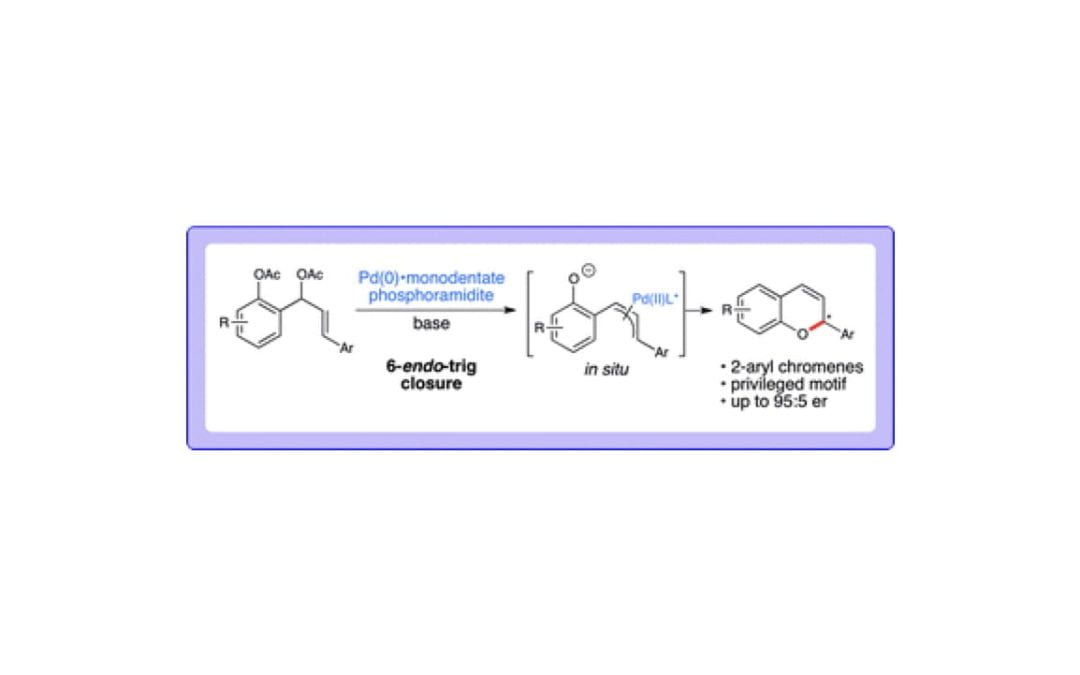
Catalytic Enantioselective Synthesis of 2-Aryl Chromenes
Zeng, B.-S.; Yu, X.; Siu, P. W.; Scheidt, K. A.
Chem. Sci. 2014, 5 (6), 2277-2281.
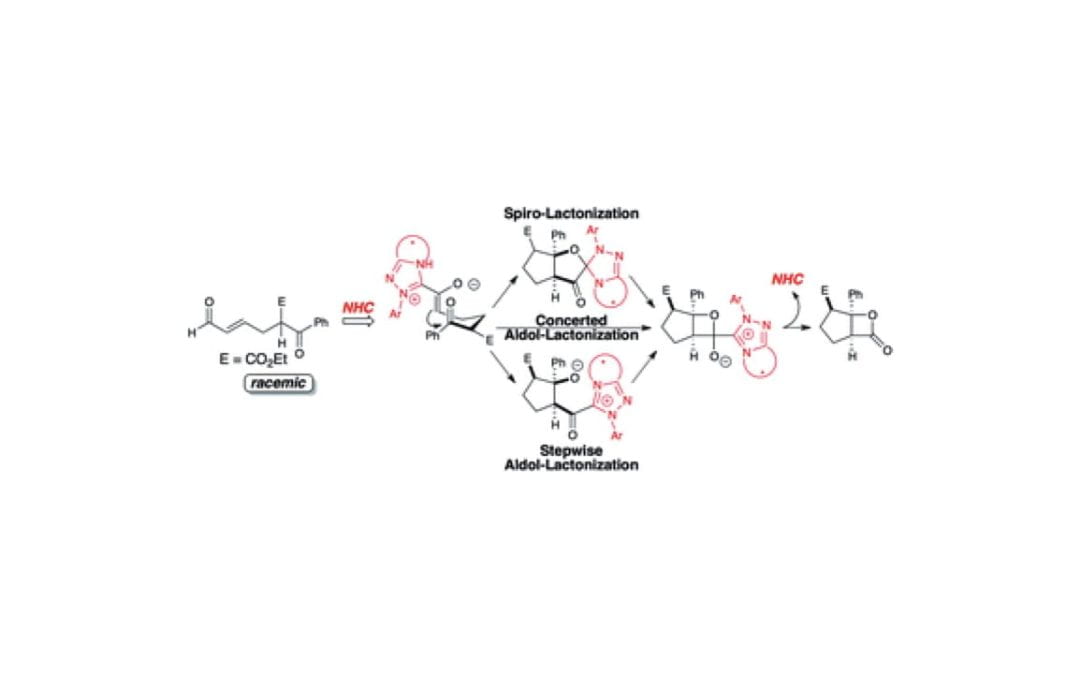
Catalytic Kinetic Resolution of a Dynamic Racemate: Highly Stereoselective β-Lactone Formation by N-Heterocyclic Carbene Catalysis
Johnston, R. C.; Cohen, D. T.; Eichman, C. C.; Scheidt, K. A.; Cheong, P.
Chem. Sci. 2014, 5, 1974-1982.
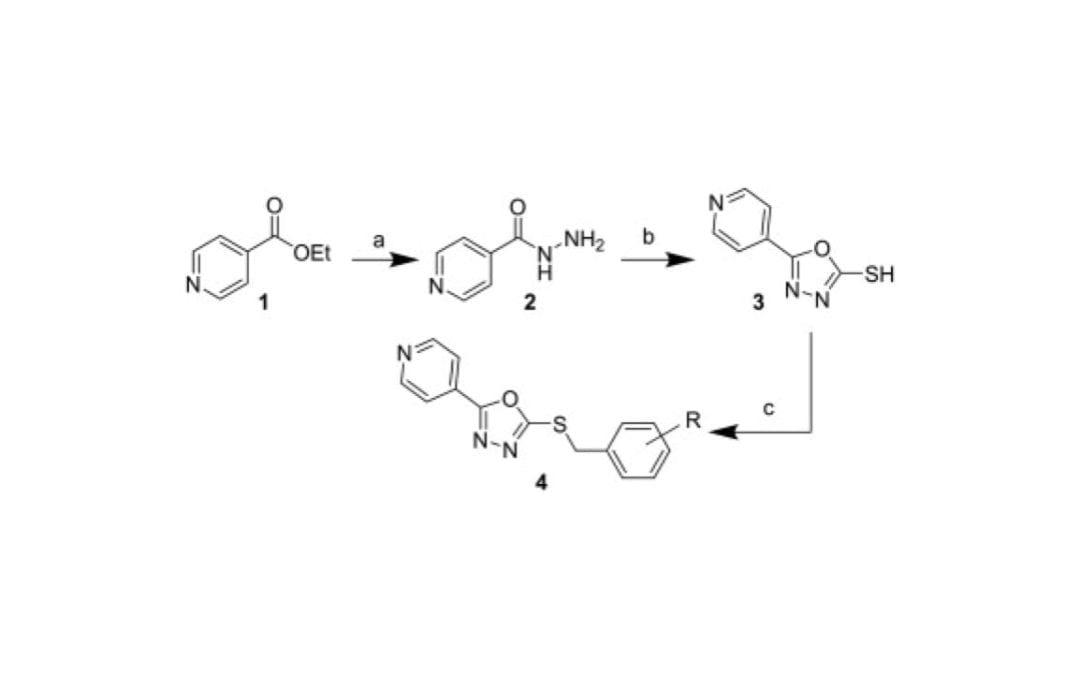
Discovery of 1,3,4-oxidiazole scaffold compounds as inhibitors of superoxide dismutase expression
Lukas, T. J.; Schiltz, G.; Arrat, H.; Scheidt, K. A.; Siddique, T.
Bioorg. Med. Chem. Lett. 2014, 24, 1532-1537.
Asymmetric Homoenolate Additions to Acyl Phosphonates through Rational Design of a Tailored N-Heterocyclic Carbene Catalyst
Jang, K. P.; Hutson, G. E.; Johnston, R. C.; McCusker, E. O.; Cheong, P. H.-Y.; Scheidt, K. A.
J. Am. Chem. Soc. 2014, 136, 76-79.
Enantioselective N-Heterocyclic Carbene Catalyzed Annulation Reactions with Imidazolidinones
McCusker, E. O.; Scheidt, K. A.
Angew. Chem. Int. Ed. 2013, 52, 13616-13620.
A Tandem Isomerization/Prins Strategy: Iridium(III)/Brønsted Acid Cooperative Catalysis
Lombardo, V. M.; Thomas, C. D.; Scheidt, K. A.
Angew. Chem Int. Ed. 2013, 52, 12910-12914.
A Mixed Dicarboxylate Strut Approach To Enhancing Catalytic Activity Of A De Novo Urea Derivative Of Metal-Organic Framework Uio-67
Siu, P. W.; Brown,Z. J.; Farha, O. K.; Hupp, J. T.; Scheidt, K. A.
Chem. Commun. 2013, 49, 10920-10922.
A facile synthesis of UiO-66, UiO-67 and their derivatives
Katz, M. J.; Brown, Z. J.; Colón, Y. J.; Siu, P. W.; Scheidt, K. A.; Snurr, R. Q.; Hupp, J. T.; Farha, O. K.
Chem. Commun. 2013, 49, 9449-9451.
A fluorescence-based thermal shift assay identifies inhibitors of mitogen activated kinase kinase 4
Krishna, S. N.; Luan, C.-H.; Mishra, R. K.; Xu, L.; Scheidt, K. A.; Anderson, W. A.; Bergan, R. C.
PLoS One 2013, 8(12).
A Dual Lewis Base Activation Strategy for Enantioselective Carbene-Catalyzed Annulations
Izquierdo, J.; Orue, A.; Scheidt, K. A.
J. Am. Chem. Soc. 2013, 135, 10634-10637.
A Concise Enantioselective Synthesis and Cytotoxic Evaluation of the Anticancer Rotenoid Deguelin Enabled by a Tandem Knoevenagel/Conjugate Addition/Decarboxylation Sequence
Farmer, R. L.; Scheidt, K. A.
Chem. Sci. 2013, 4 (8), 3304-3309.
A Zwitterionic Metal-Organic Framework with Free Carboxylic Acid Sites that Exhibits Enhanced Hydrogen Adsorption Energies
Lalonde, M. B.; Getman, R.; Lee, J. Y.; Roberts, J.; Sarjeant, A.; Scheidt, K. A.; Georgiev, P. A.; Embs, J. P.; Eckert, J.; Farha, O. K.; Snurr, R. Q.; Hupp, J. T.
CrystEngComm. 2013, 15, 9408-9414.
A Continuum of Progress: Applications of N-Hetereocyclic Carbene Catalysis in Total Synthesis
Izquierdo, J.; Hutson, G. E.; Cohen, D. T.; Scheidt, K. A.
Angew. Chem. Int. Ed. 2012, 51, 11686-11698.
N-Heterocyclic Carbene-Like Catalysis by a Metal-Organic Framework (MOF) Material
Lalonde, M. B.; Farha, O. K.; Scheidt, K. A.; Hupp, J. T.
ACS Catalysis 2012, 2 (8), 1550–1554.
Assembly of Four Diverse Heterocyclic Libraries Enabled by Prins Cyclization, Au-Catalyzed Enyne Cycloisomerization, and Automated Amide Synthesis
Cui, J.; Chai, D. I.; Miller, C.; Hao, J.; Thomas, C.; Wang, J.; Scheidt, K. A.; Kozmin, S. A.
J. Org. Chem. 2012, 77, 7435–7470.
Acyloin Coupling Reactions
O’Bryan, E. A.; Scheidt, K. A.
Comprehensive Organic Synthesis II. Vol 3, Marek, I., Knochel, P. eds. 2012, 621-655.
Catalytic Dynamic Kinetic Resolutions with N-Heterocyclic Carbenes: Asymmetric Synthesis of Highly Substituted β-Lactones
Cohen, D. T.; Eichman, C. C.; Phillips, E. M.; Zarefsky, E. R.; Scheidt, K. A.
Angew. Chem. Int. Ed. 2012, 51, 7309–7313.
N-Heterocyclic Carbene-Catalyzed Aldol Desymmetrizations
Scheidt, K. A.; Phillips, E. M.; Dugal-Tessier, J. in Asymmetric Synthesis- More Methods and Applications, Eds. Christmann and Bräse. Wiley-VCH, 2012.
An N-Heterocyclic Carbene/Lewis Acid Strategy for the Stereoselective Synthesis of Spirooxindole Lactones
Dugal-Tessier, J.; O’Bryan, E. A.; Schroeder, T. B. H.; Cohen, D. T.; Scheidt, K. A.
Angew. Chem. Int. Ed. 2012, 51, 4963-4967.
Carbene Catalysis: Beyond the Benzoin and Stetter Reactions
Cardinal-David, B.; Scheidt, K. A.
in Inventing Reactions: Topics in Organometallic Chemistry, Ed. Goosen. Springer, 2012.
Urea Metal–Organic Frameworks as Effective and Size Selective Hydrogen–Bond Catalysts
Roberts, J. M.; Fini, B. M.; Sarjeant, A. A.; Farha, O. K.; Hupp, J. T.; Scheidt, K. A.
J. Am. Chem. Soc. 2012, 134, 3334-3337.
Lewis Base-Promoted Carbon-Carbon sp3–sp3 Coupling Reactions of α-Silyl Silylethers
Brekan, J. A.; Chernyak, D.; White, K. L.; Scheidt, K. A.
Chem. Sci. 2012, 3, 1205-1210.
Asymmetric Methods for the Synthesis of Flavanones, Chromanones, and Azaflavanones
Nibbs, A. E.; Scheidt, K. A.
Eur. J. Org. Chem. 2012, 3, 449-462.